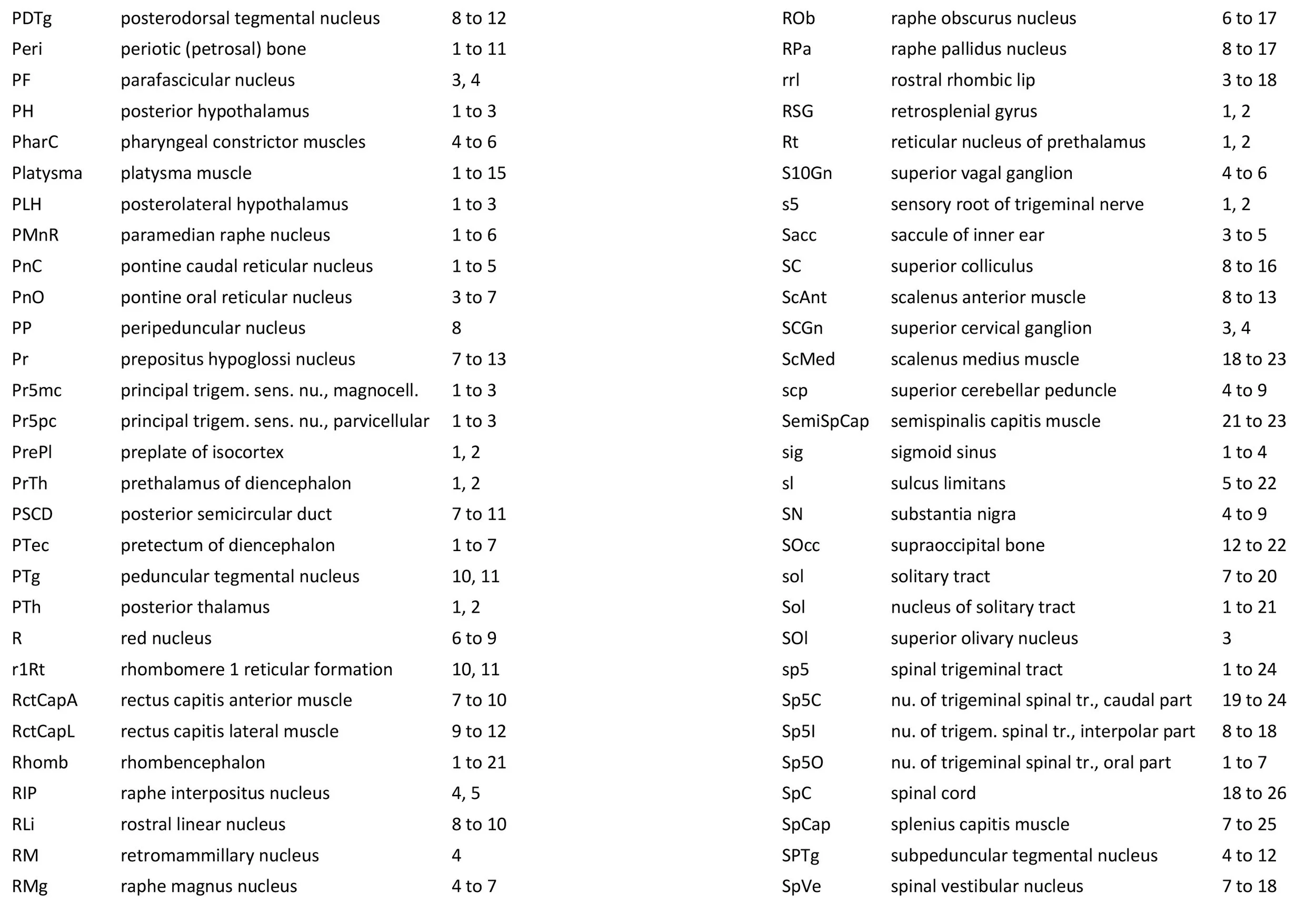
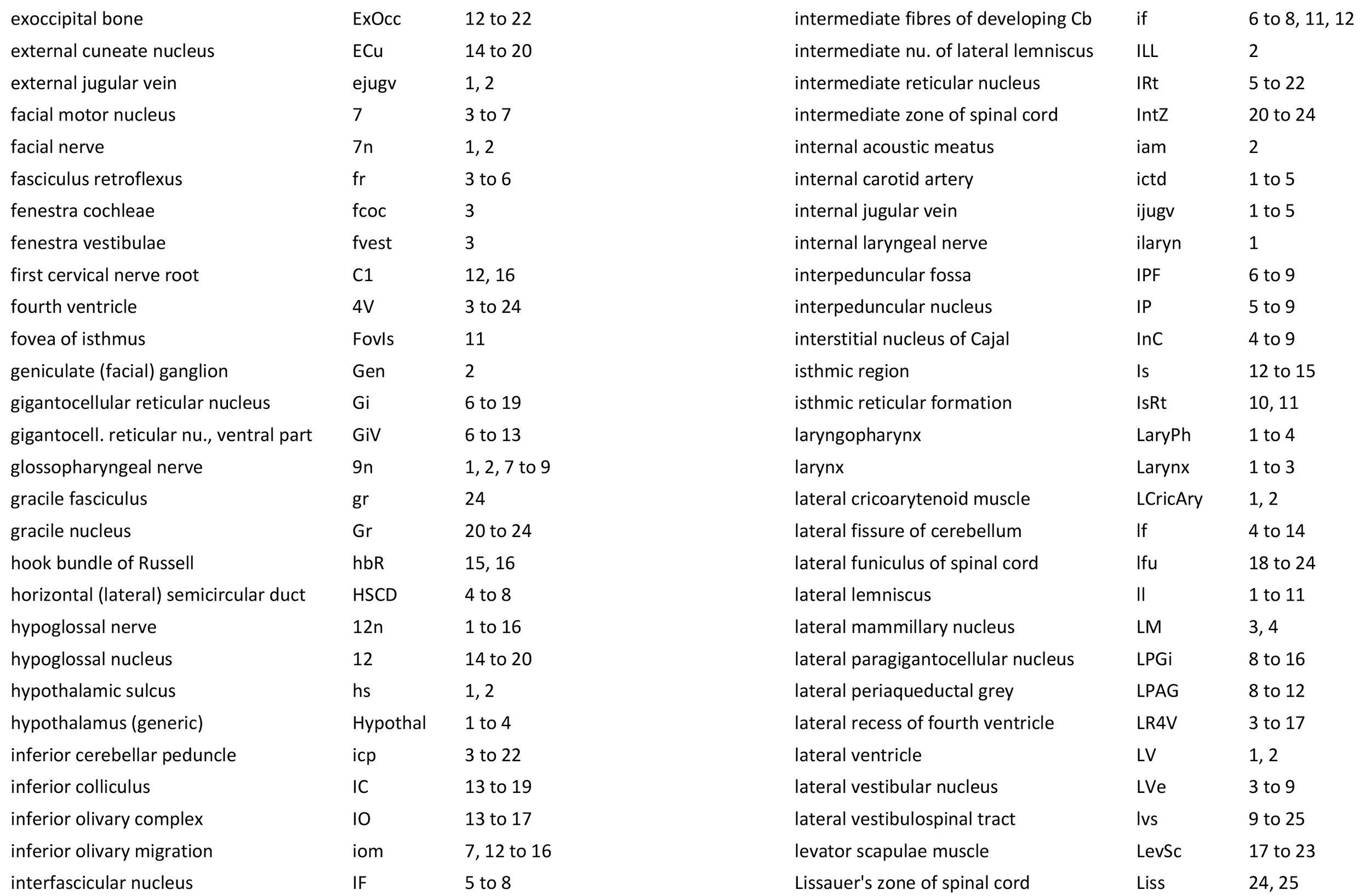
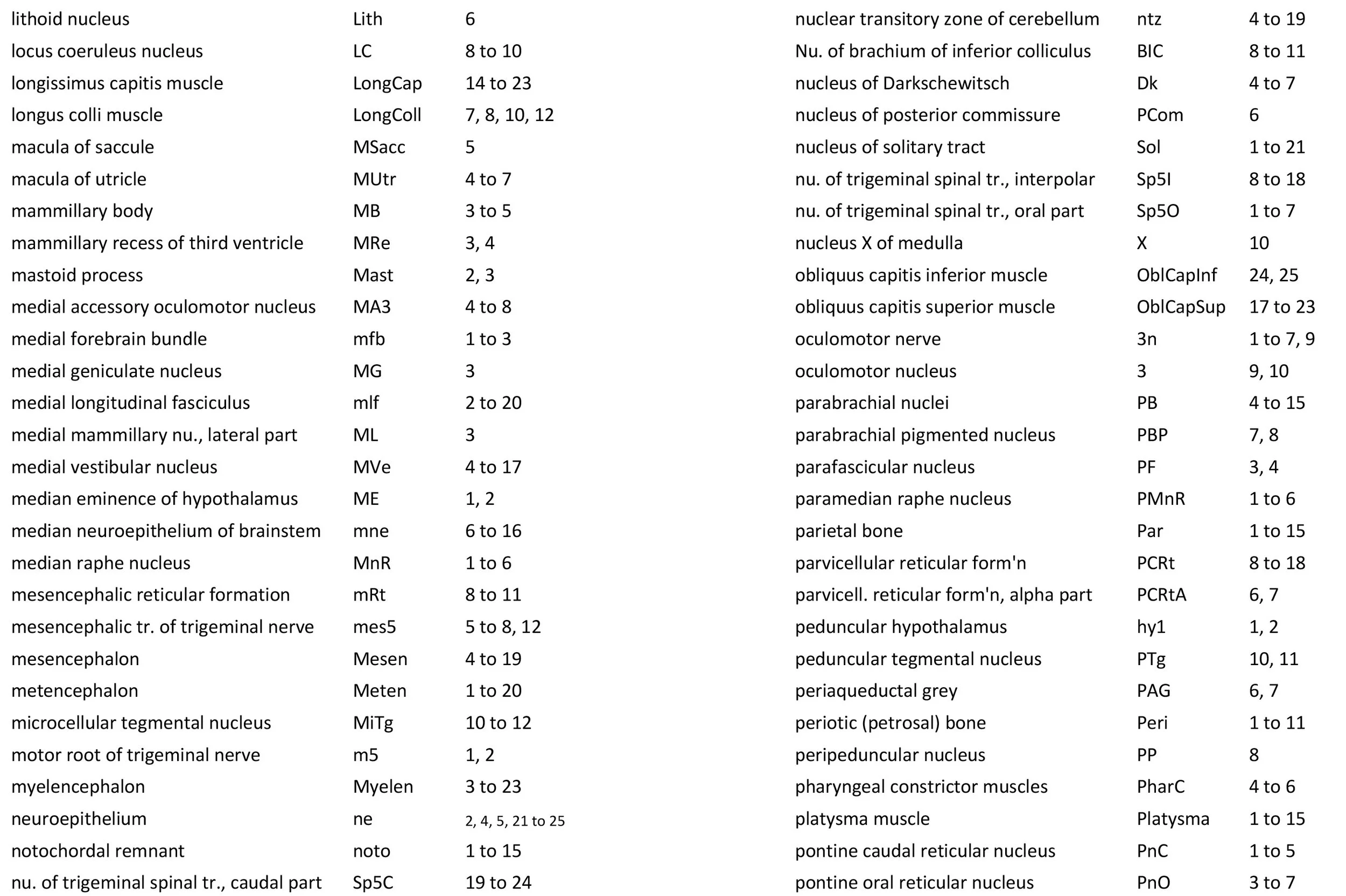
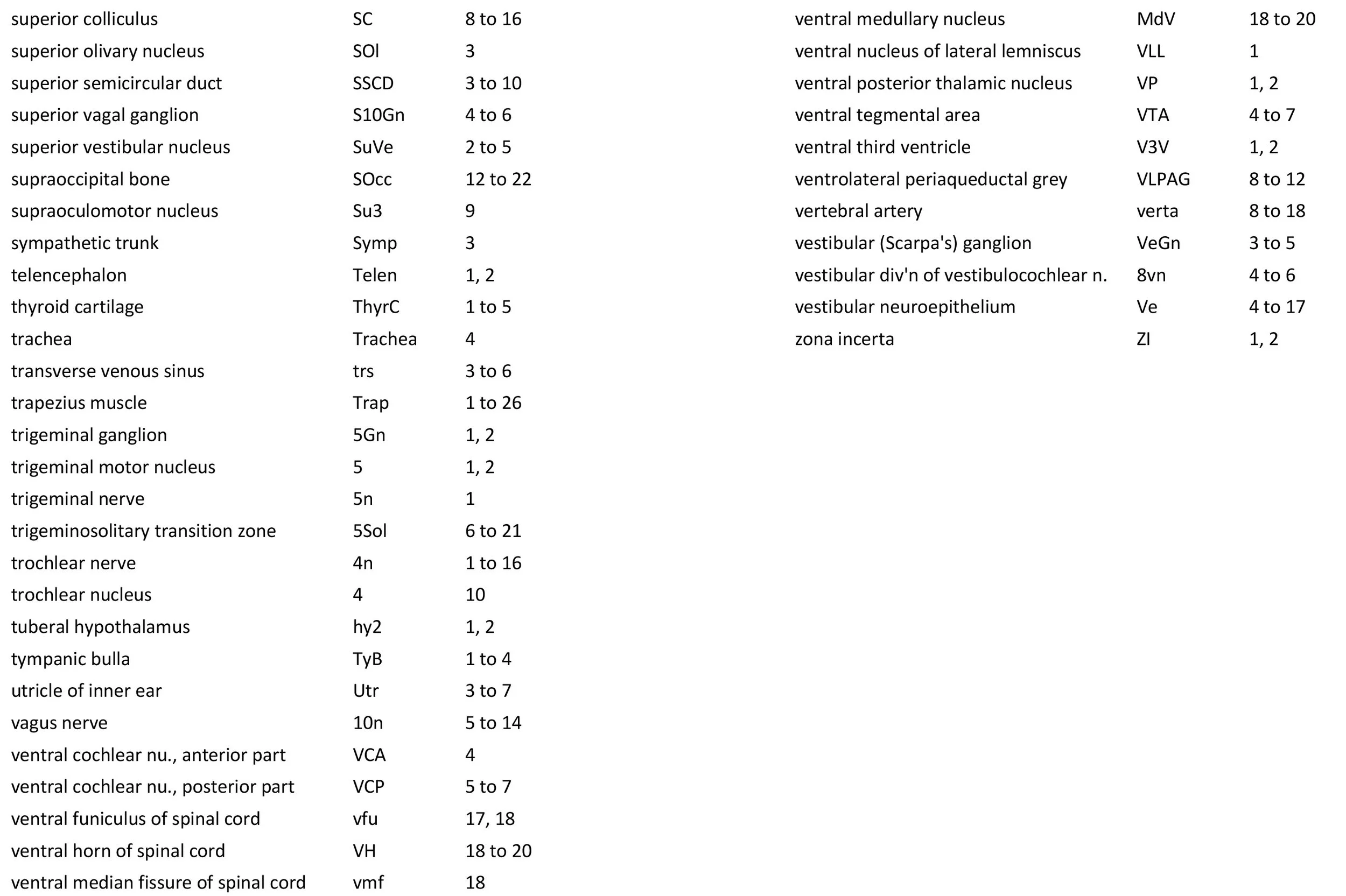
Atlas of the Brainstem and Posterior Head of a Newly Hatched (16.75 mm GL) Platypus (M44)
Introduction
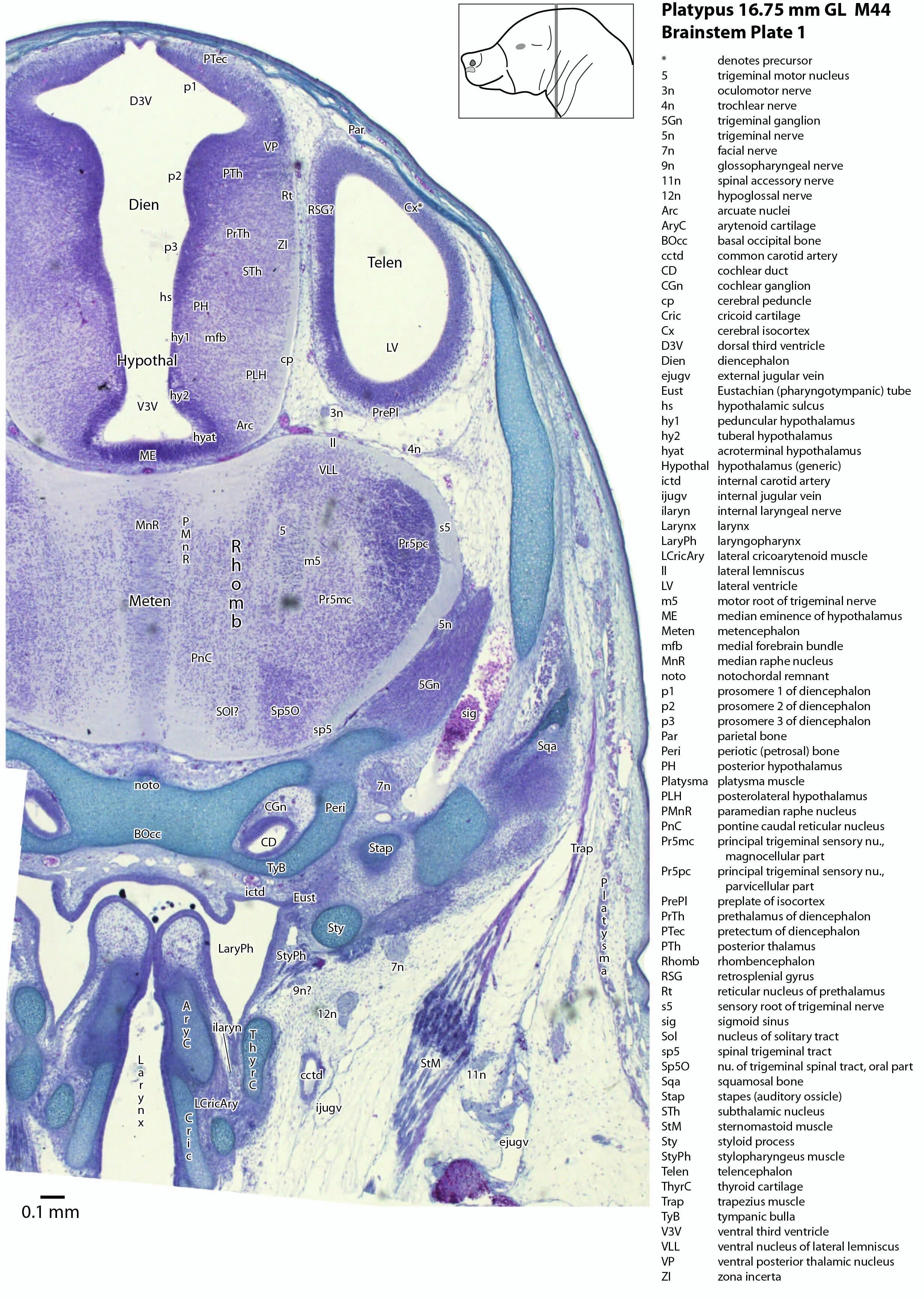
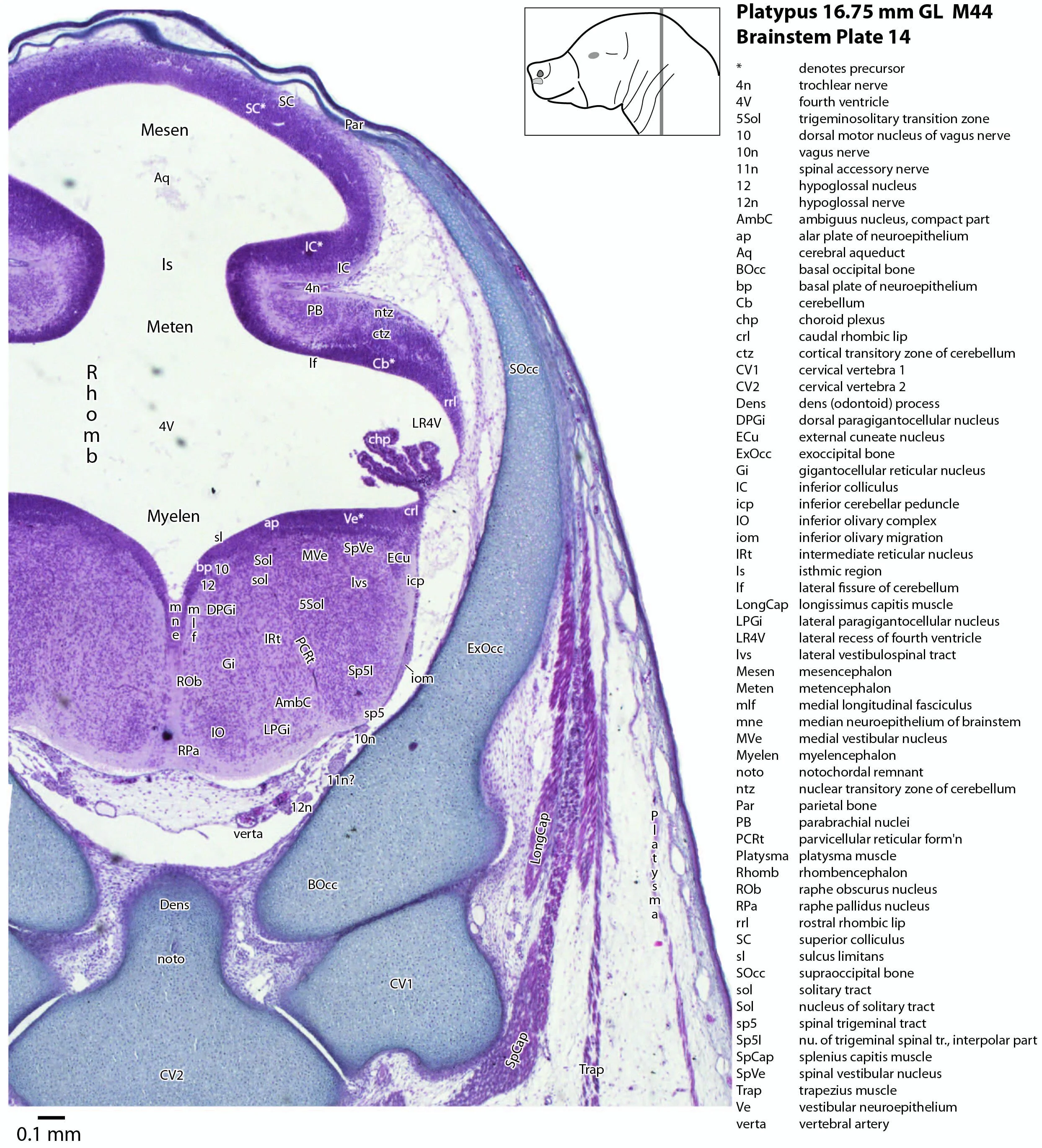
This series of images illustrates the extent of brainstem development at hatching and shows the large size of the behaviourally important trigeminal sensory nuclear complex (in particular Pr5mc, Pr5pc, Sp5O, Sp5I, Sp5C) that serves the platypus bill.
A platypus of 16.75 mm GL (greatest length) would be no more than 1 day after hatching (Ashwell, 2013), where hatching occurs at about 14 to 16 mm GL.
Details on platypus biology can be found at the Platypus page of this website.
Methods
The specimen illustrated here is M44 (a newly hatched platypus) of the Hill collection, stored at the Museum für Naturkunde in Berlin. The most likely site of collection is eastern New South Wales, near Sydney. Note that the specimen card states 16.45 mm GL (newly hatched), but the slides state 16.75 mm GL.
The specimen had been collected in the late 19th century by JP Hill, embedded in paraffin wax and sectioned transversely (frontally) at 10 µm thickness. The stain was not identified on the specimen card, but it appears to be a variant of hematoxylin stain.
Sections at approximately 70 µm intervals were photographed with the aid of a Zeiss Axioplan2 fitted with an AxioCamMRc5 camera. All sections were photographed with a 2.5x objective and images were calibrated by photographing a scale bar at the same magnification. Images were placed in Adobe Illustrator 2021 and delineated.
General observations
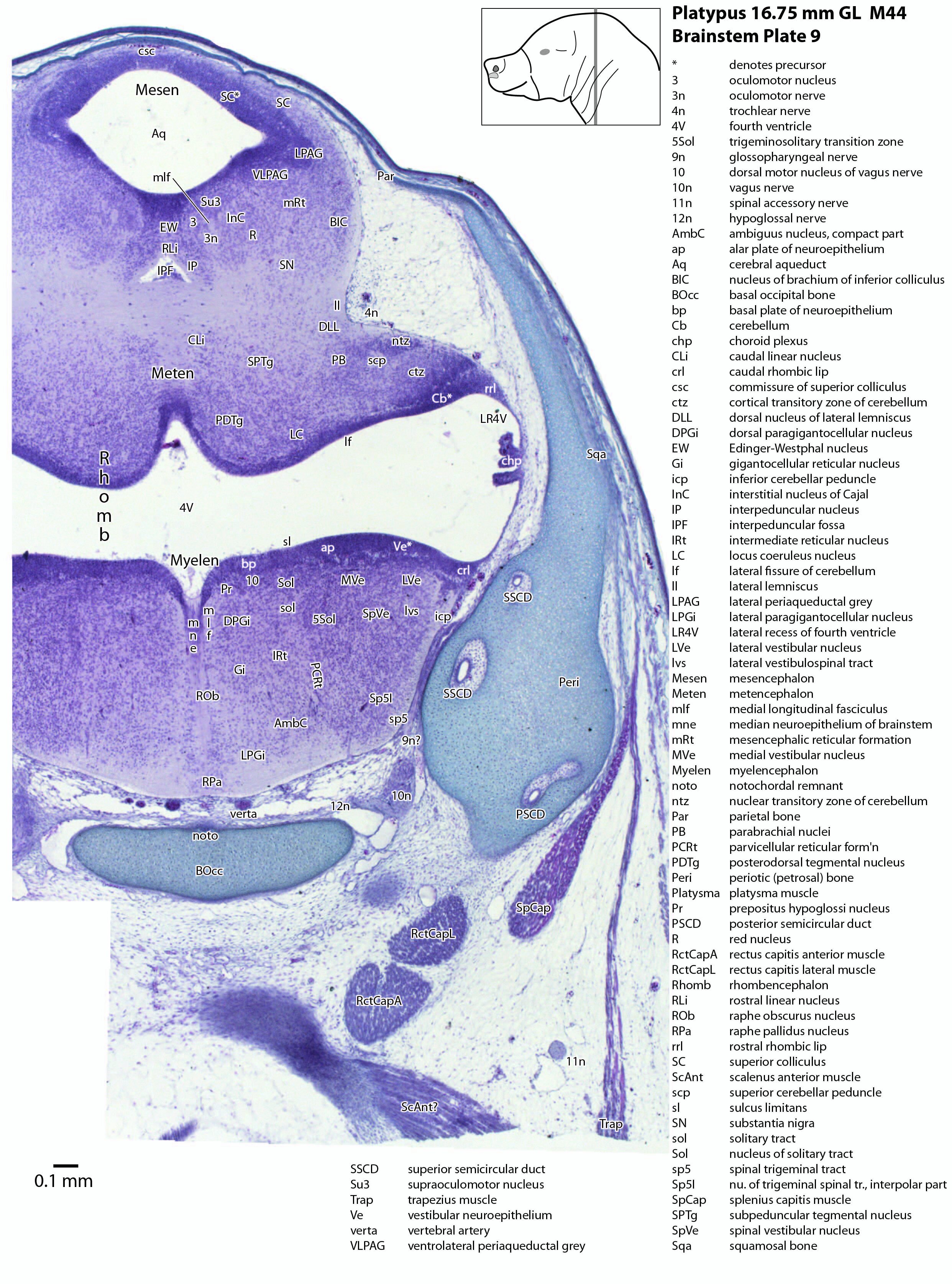
Brainstem development at early post-hatching is much more advanced than cortical development, indicating that the limited behavioural repertoire of the young platypus (e.g. locating the maternal milk patch) is mediated solely through brainstem and spinal cord circuitry at this age. Compare the poor differentiation of the cerebral isocortex (Cx in plate 1) with the large areas of postmitotic neurons in the brainstem in the same plate, e.g. the trigeminal sensory nuclei (see below).
Mesencephalon (midbrain) and isthmus
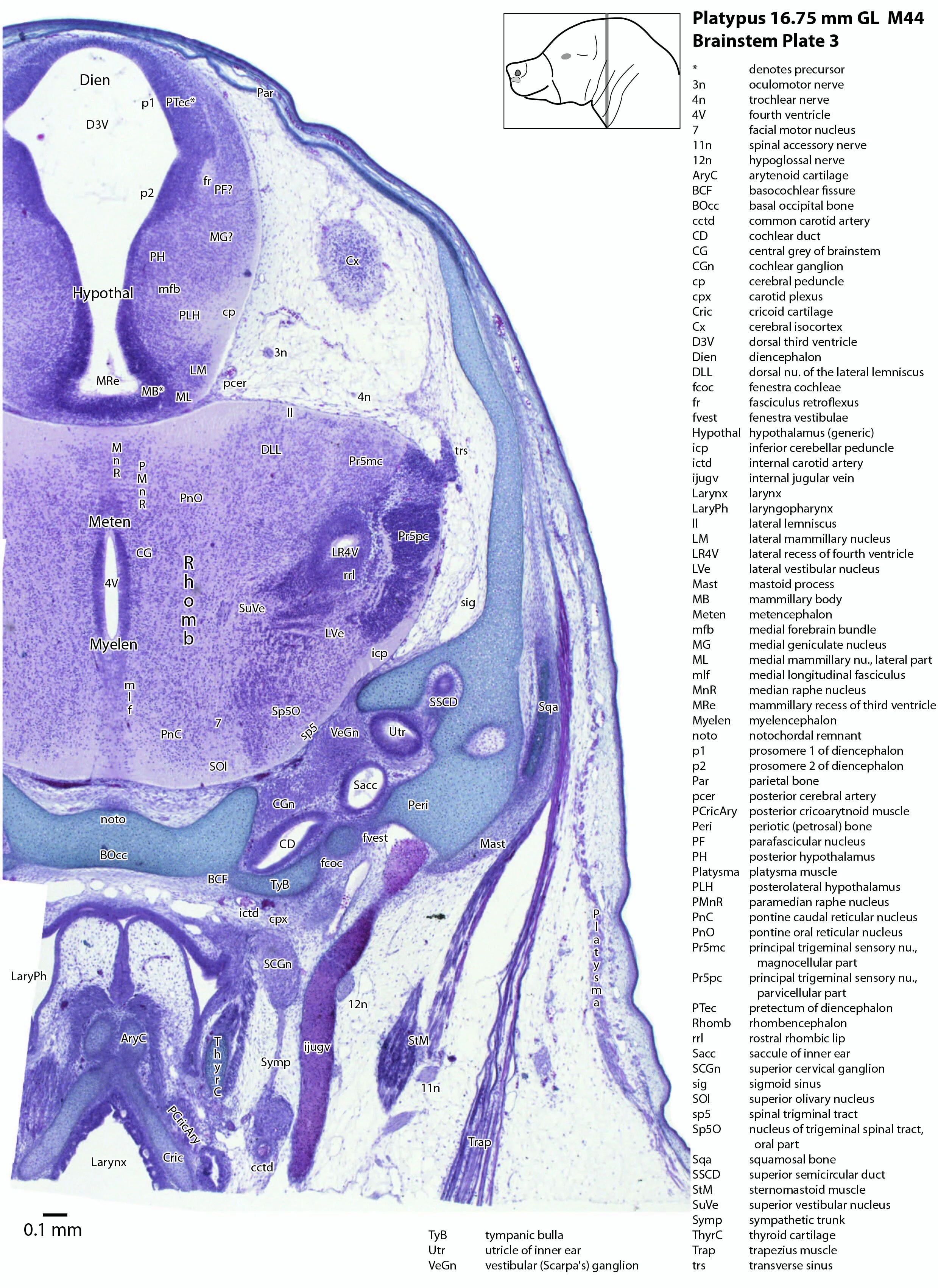
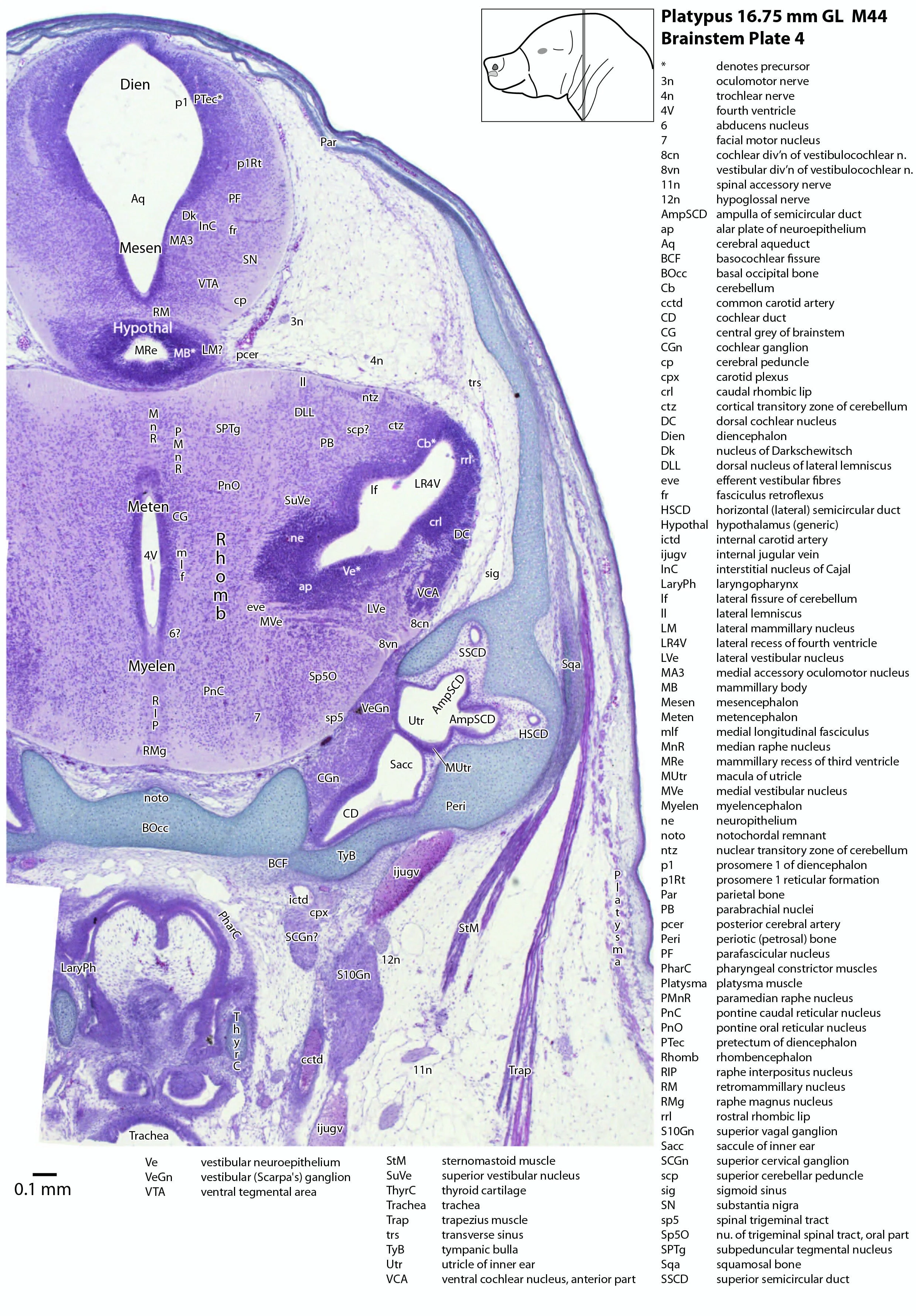
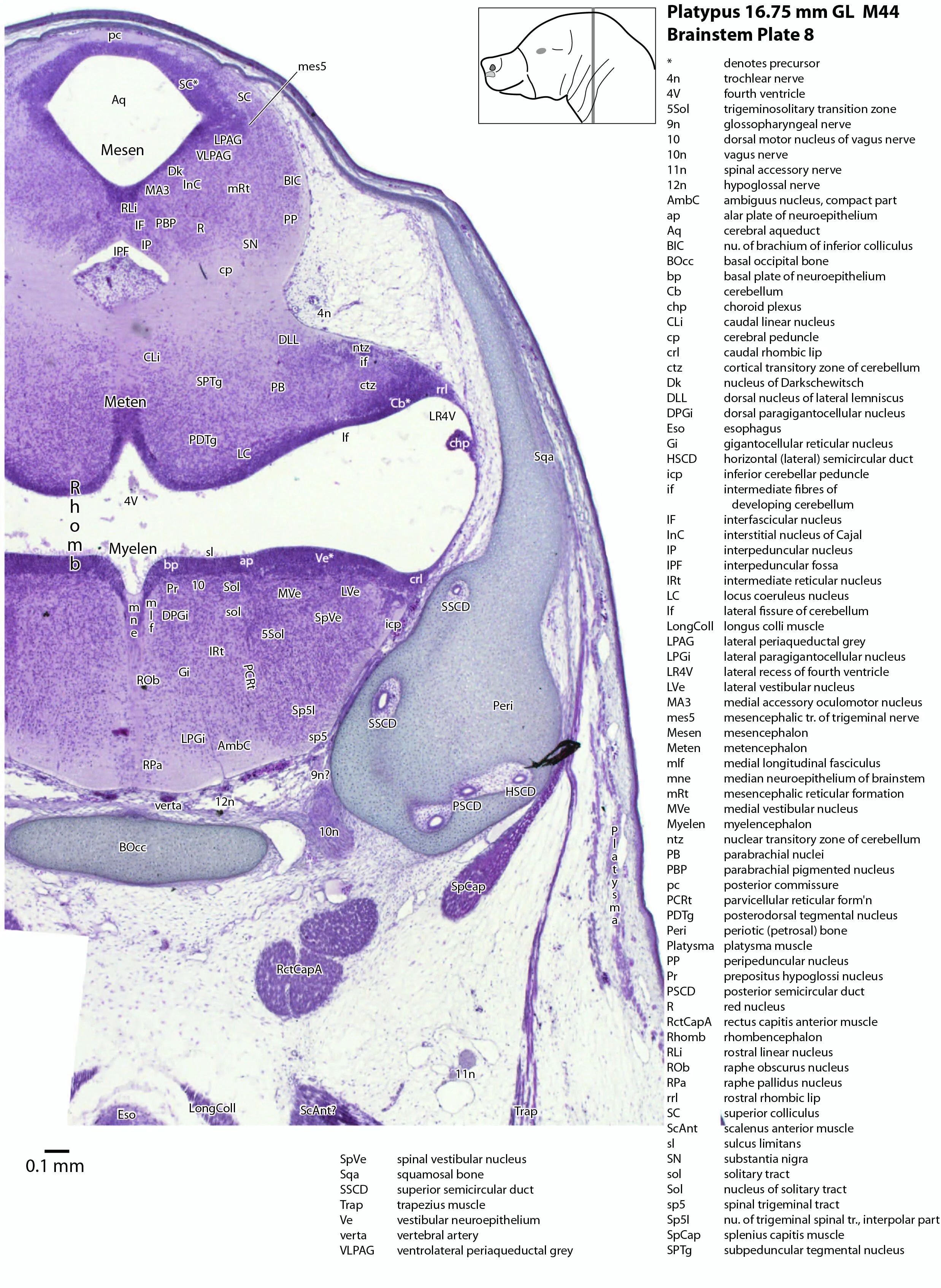
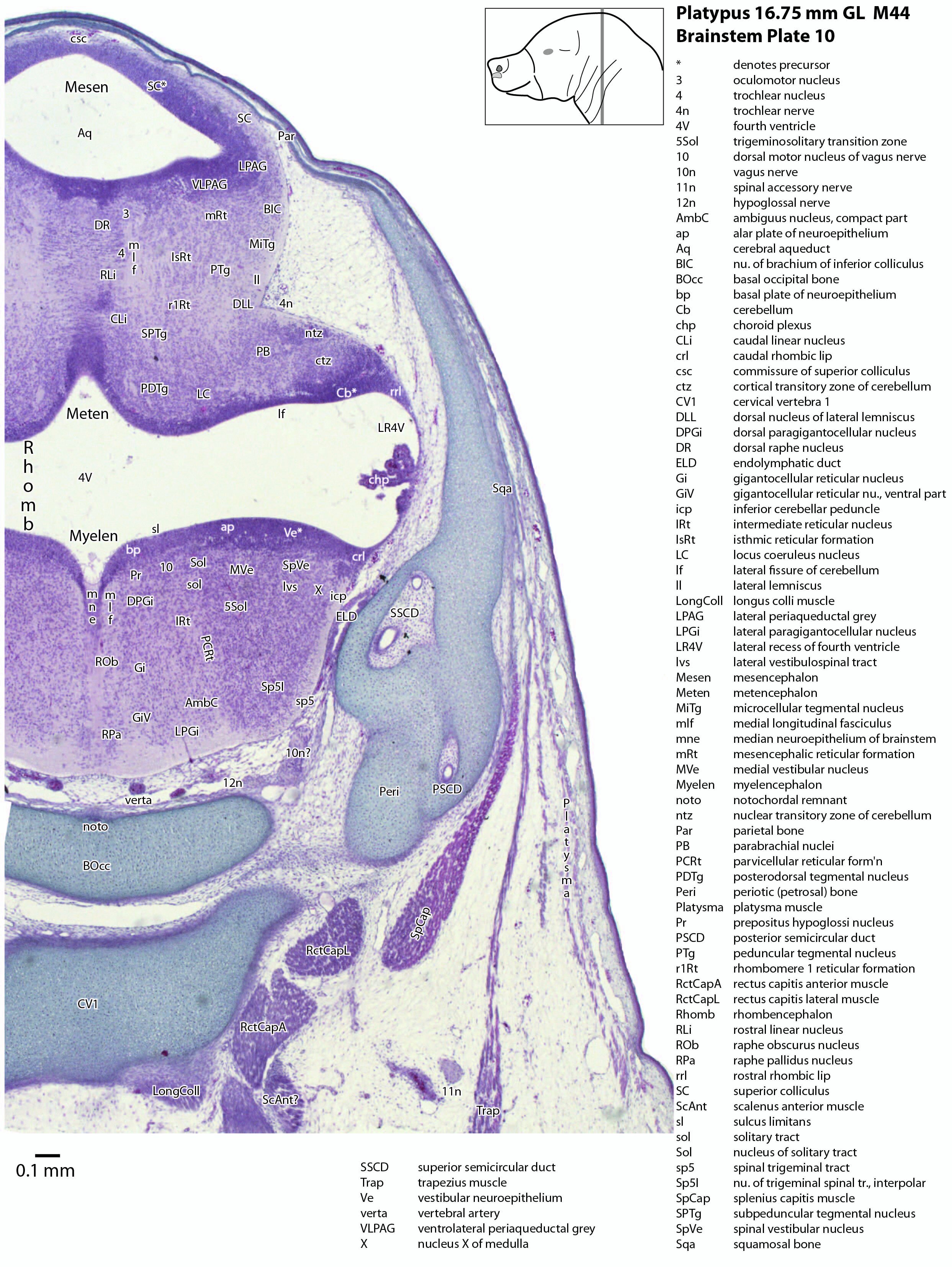
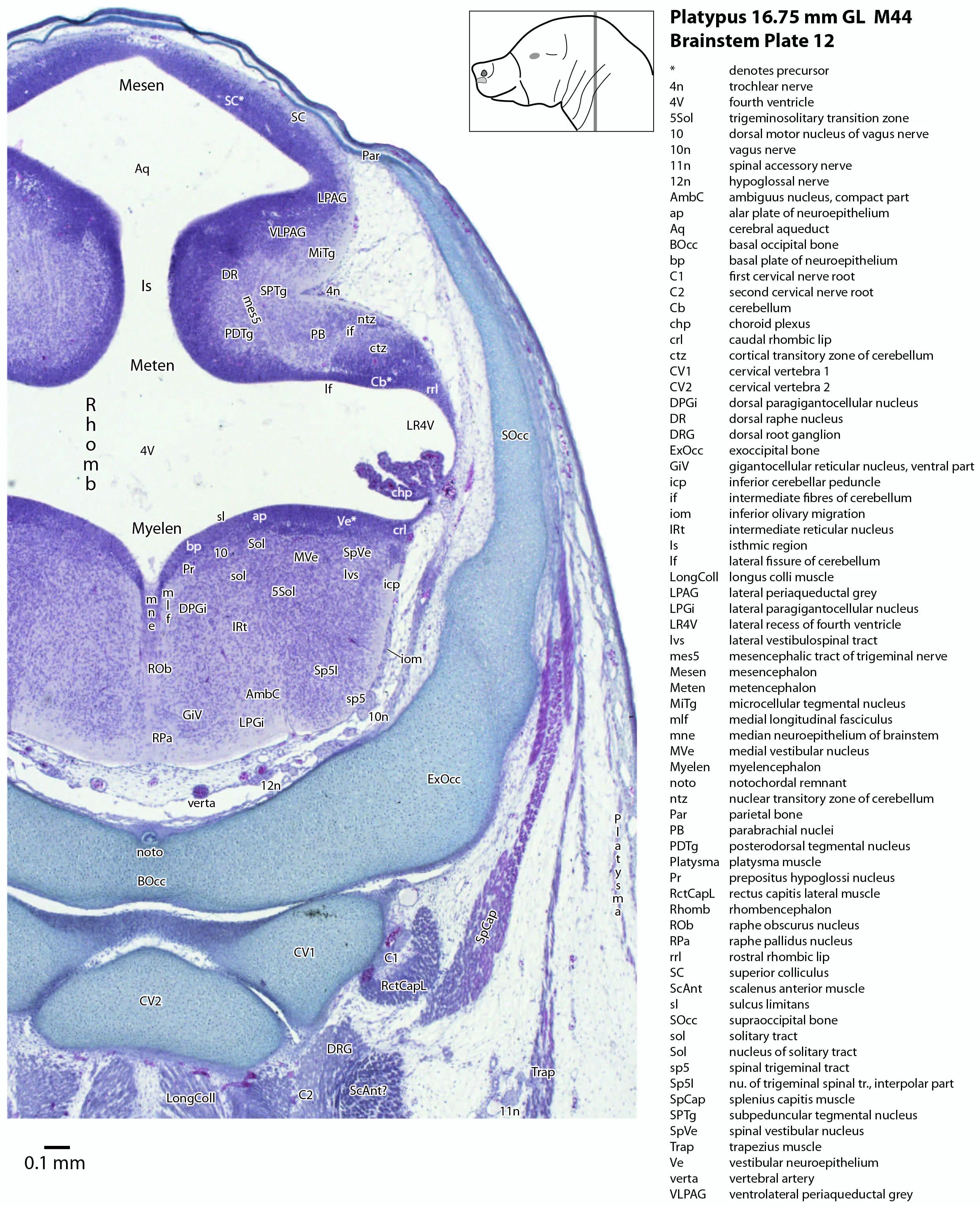
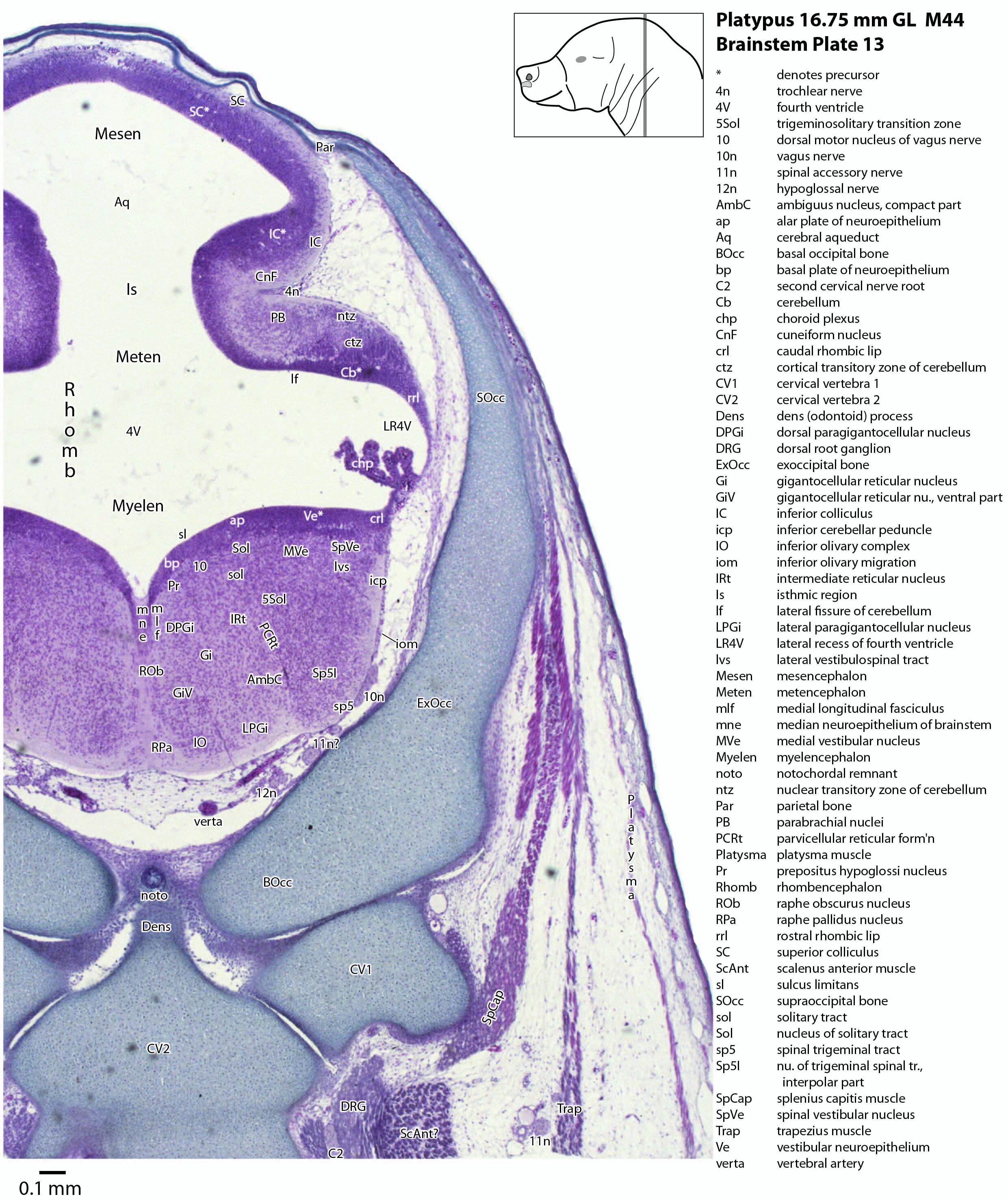
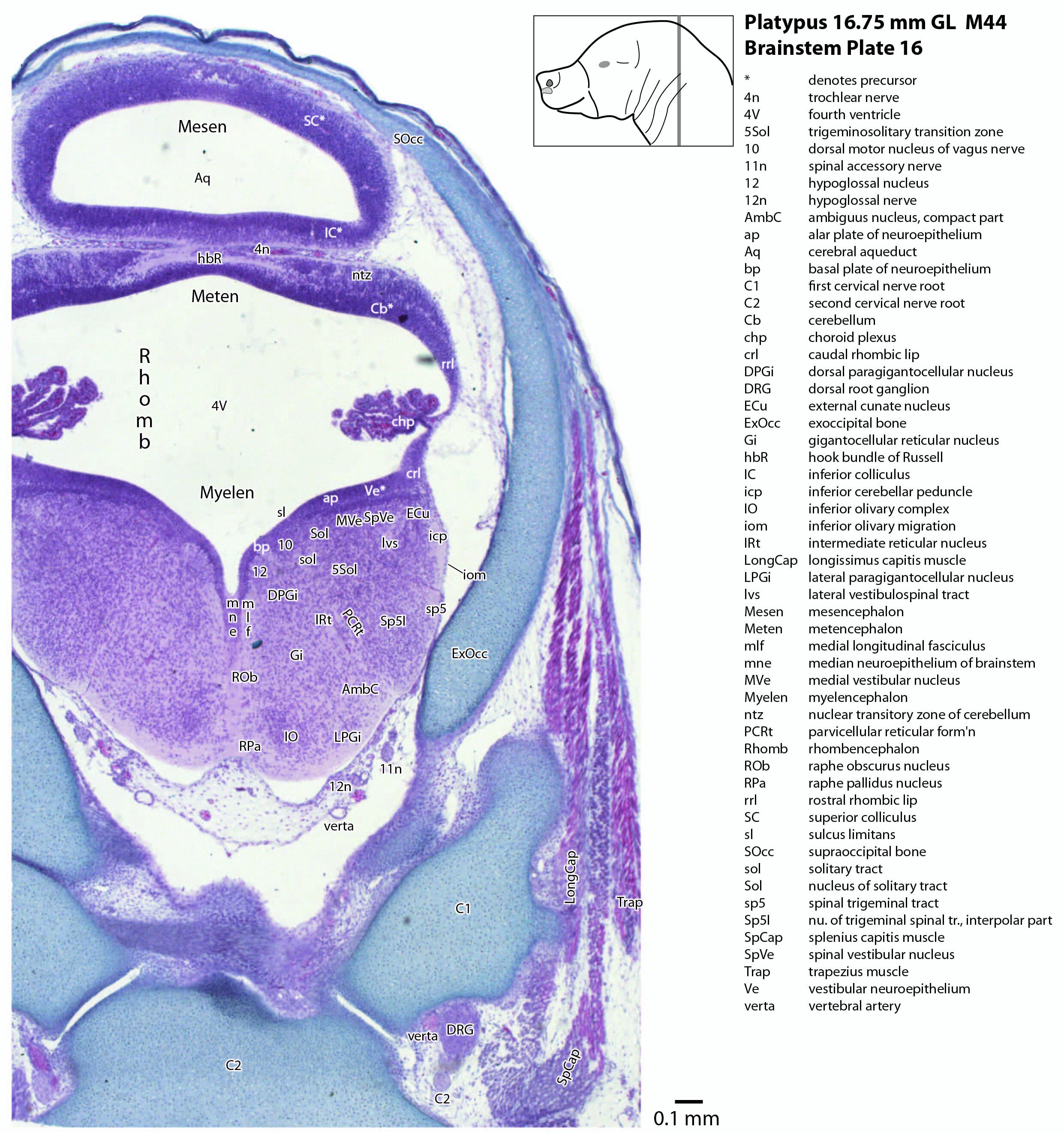
The midbrain is divided into a roof (tectum) and a ventral tegmentum. The ventricular system cavity of the midbrain is still large (cerebral aqueduct, Aq in plates 4 to 18) and the tectum is dominated by a large proliferative compartment for the superior colliculus (SC*) with only a thin layer of postmitotic neurons overlying the proliferative zone (plats 8 to 16). The most caudal part of the dorsal midbrain is formed by the proliferative zone for the developing inferior colliculus (IC*, plates 13 to 19). Differentiation of the midbrain tegmentum has proceeded sufficiently that the red nucleus (R in plates 6 to 9) and substantia nigra (SN in plates 4 to 9) are visible. A large fibre-rich cerebral peduncle (cp in plates 1 to 8, more accurately called the basis pedunculi) is visible, suggesting some longitudinal connections are already present between the diencephalon and brainstem.
The isthmus (Is in plates 12 to 15) is a small region between the mesencephalon and rhombencephalon. It is marked by a narrowing of the ventricular system lumen (FovIs in plate 11) between the cerebral aqueduct and the fourth ventricle.
Rhombencephalon terminology
The classical division of the rhombencephalon (hindbrain vesicle) into metencephalon and myelencephalon is not strictly valid given modern concepts of rhombomeric subdivision based on gene expression (Watson, 2012), but the terms met- and myel/encephalon persist in embryology textbooks and so have been used here.
Rhombic lip
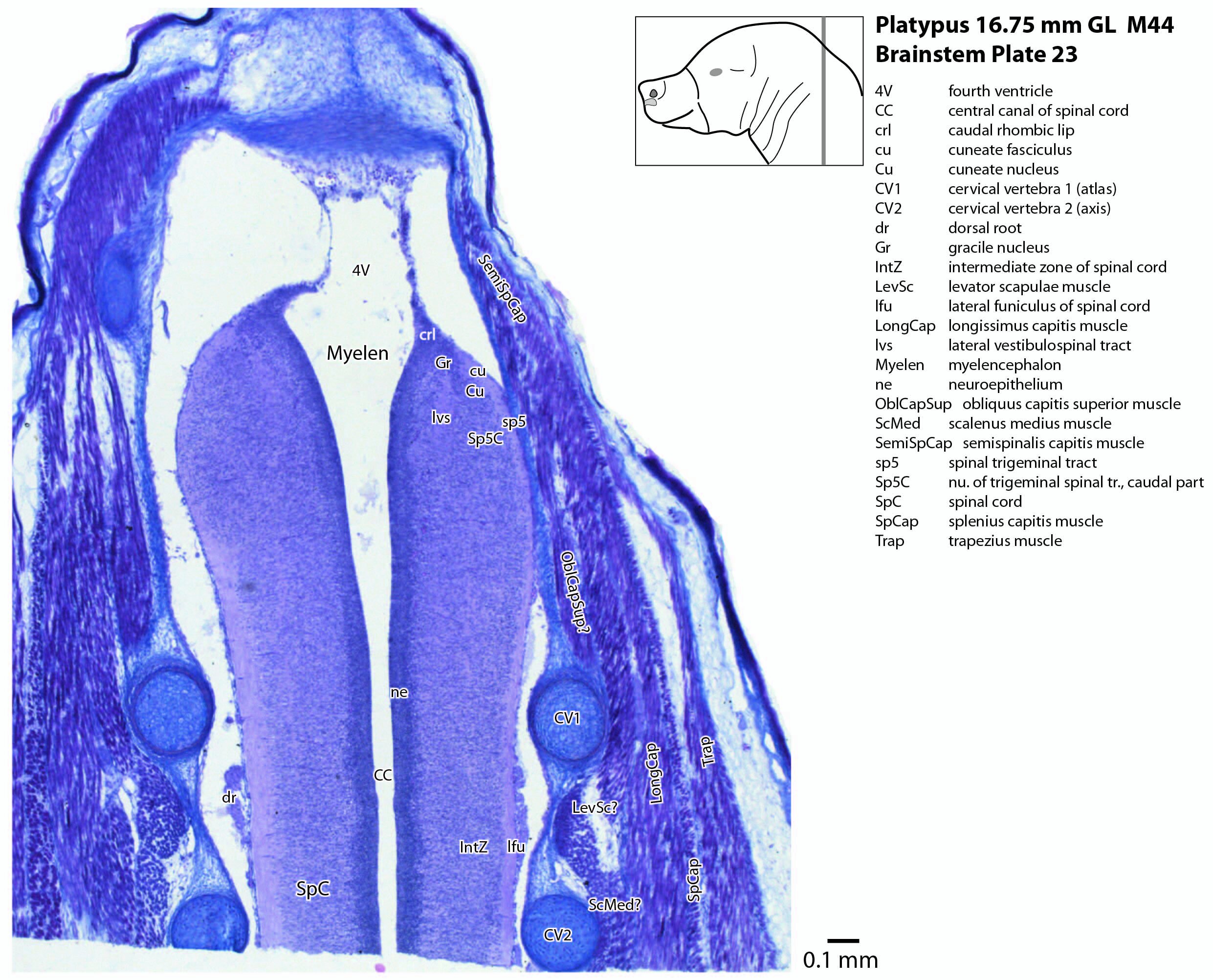
The rhombic lip is a developmentally important proliferative region at the margin of the fourth ventricle. The rostral rhombic lip (rrl in plates 3 to 18) is an important source of microneurons for the cerebellum, whereas the caudal rhombic lip (crl in plates 4 to 24) produces microneurons for the ventral brainstem (e.g. for Pr5pc and precerebellar nuclei like the inferior olive, IO in plates 13 to 17).
Cerebellum
The developing cerebellum arises from the rostral rhombencephalon. It consists of a ventricular neuroepithelium (Cb* in plates 4 to 20) adjacent to the rostral rhombic lip (rrl in plates 3 to 18), with some postmitotic neurons grouped into a superficial nuclear transitory zone (ntz in plates 4 to 19) and a deeper cortical transitory zone (ctz in plates 4 to 15). The external granular layer has not appeared yet, but some putative fibre bundles associated with the cerebellum can be seen. These include the superior cerebellar peduncle (scp in plates 4 to 9), the inferior cerebellar peduncle (icp in plates 3 to 22) and the hook bundle of Russell (hbR, putative fastigial nucleus efferents, in plates 15, 16).
Ventral rhombencephalon derivatives: “pons” and medulla oblongata
As for all vertebrates, the rhombencephalon is organised into functionally distinct radial wedges fanning out from the basal (motor neuroepithelium) and alar (sensory neuroepithelium) plates. The most medial neuronal group is the raphe nuclei (MnR, PMnR, RMg, ROb, RPa), flanked in succession by a large neuronal (gigantocellular) region (DPGi, Gi, GiV, LPGi in plates 6 to 19), an intermediate (autonomic) zone (IRt in plates 5 to 22), a parvicellular zone (PCRtA and PCRt in plates 6 to 18), and finally a group of sensory columns (trigeminal and solitary nuclei medially, vestibular and cochlear nuclei laterally).
Trigeminal sensory nuclei
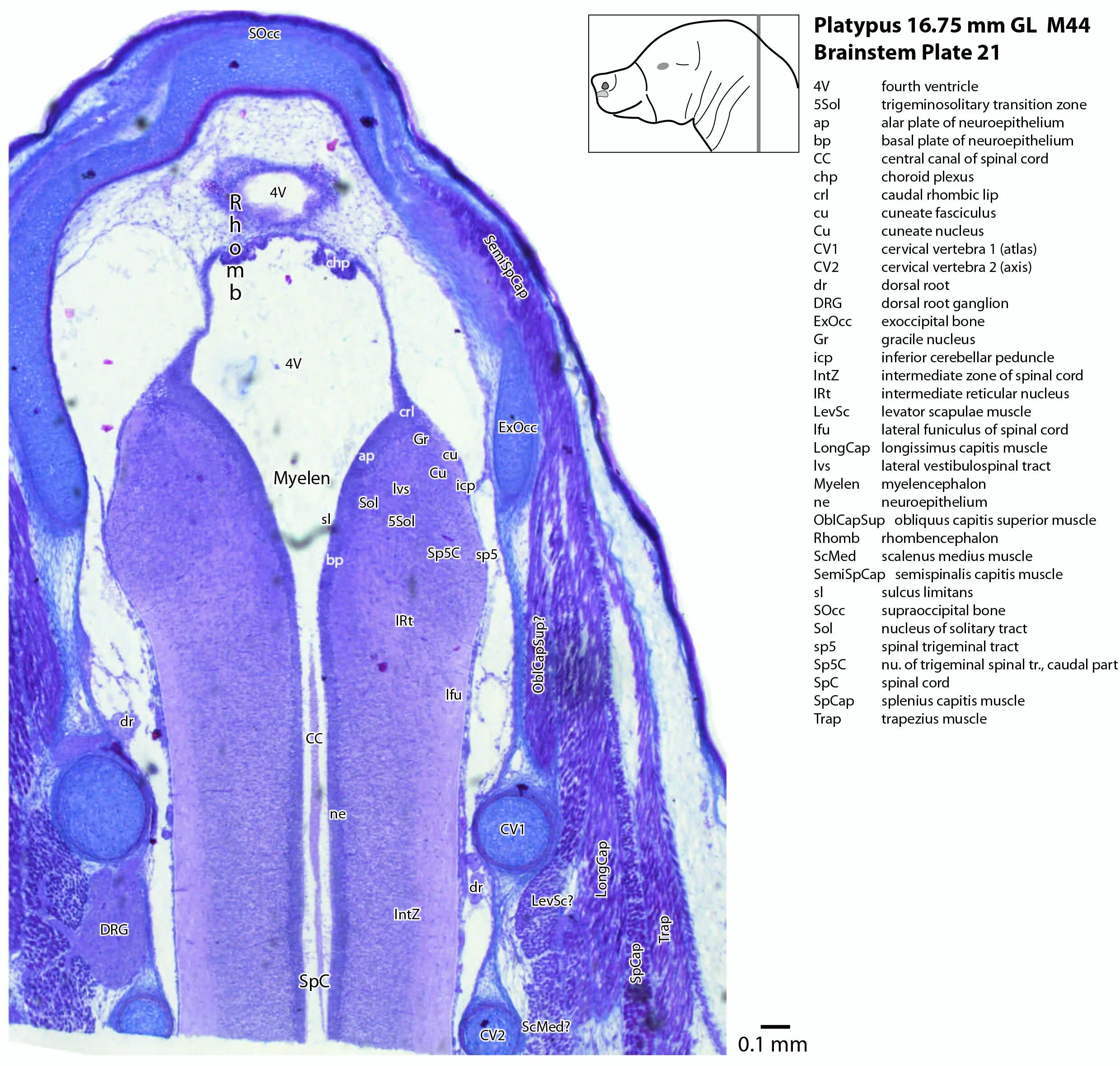
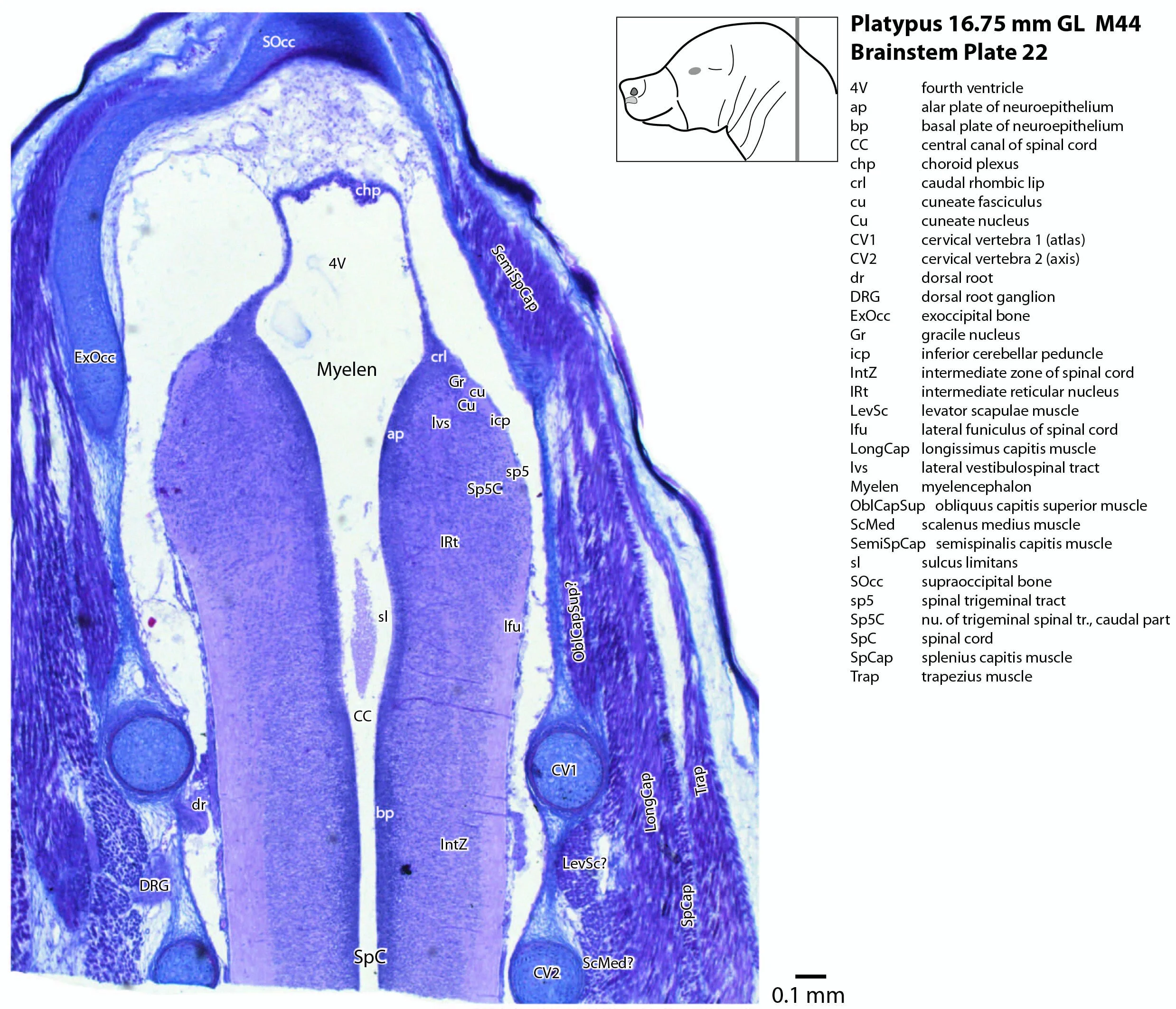
The most prominent feature of the brainstem is the trigeminal sensory column, consisting of a very large principal trigeminal sensory nucleus rostrally, with magnocellular (Pr5mc in plates 1 to 3) and parvicellular (Pr5pc in plates 1 to 3) components. More caudal parts of the trigeminal sensory complex include (in rostrocaudal sequence) oral, interpolar and caudal parts of the nucleus of trigeminal spinal tract (respectively, Sp5O in plates 1 to 7, Sp5I in plates 8 to 18, Sp5C in plates 19 to 24).
Inner ear
Most components of the otocyst/inner ear are visible. These include the cochlear duct (plates 1 to 4), utricle (plates 3 to 7), saccule (plates 3 to 5), semicircular ducts (plates 3 to 10) and endolymphatic duct and sac (plates 6 to 11). Sensory maculae of the utricle and saccule are also visible (plates 4 to 7). The cochlear and vestibular ganglia (CGn in plates 1 to 4; VeGn in plates 3 to 5) have separated from the facial (geniculate) ganglion (Gen in plate 2).
Cranial nerves
Cranial nerves 3 (oculomotor) and 4 (trochlear) can be traced from the mesencephalon and isthmus (respectively) forward (oculomotor in plates 1 to 9; trochlear in plates 1 to 16). Cranial nerve 7 (facial) can be traced through the middle ear cavity and out the stylomastoid foramen (plates 1 and 2). Cranial nerves 9, 10 and 11 (glossopharyngeal, vagus and accessory, respectively) are found alongside the brainstem before emerging from the chondrocranium. The glossopharyngeal nerve is best found adjacent to the sole muscle it supplies, the stylopharyngeus (plates 1 and 2). The vagus nerve (10n in plates 5 to 14) can be traced through its superior vagal ganglion (S10Gn in plates 4 to 6) and is closely associated with the common carotid artery and internal jugular vein. The (spinal) accessory nerve (plates 1 to 20) arises from the most caudal medulla and rostral spinal cord and passes forward to lie alongside the vagus nerve. It is easiest to identify outside the chondrocranium alongside the two muscles it supplies, sternomastoid (StM in plates 1 to 4) and the cranial part of trapezius (Trap in plates 1 to 26).
Spinal cord
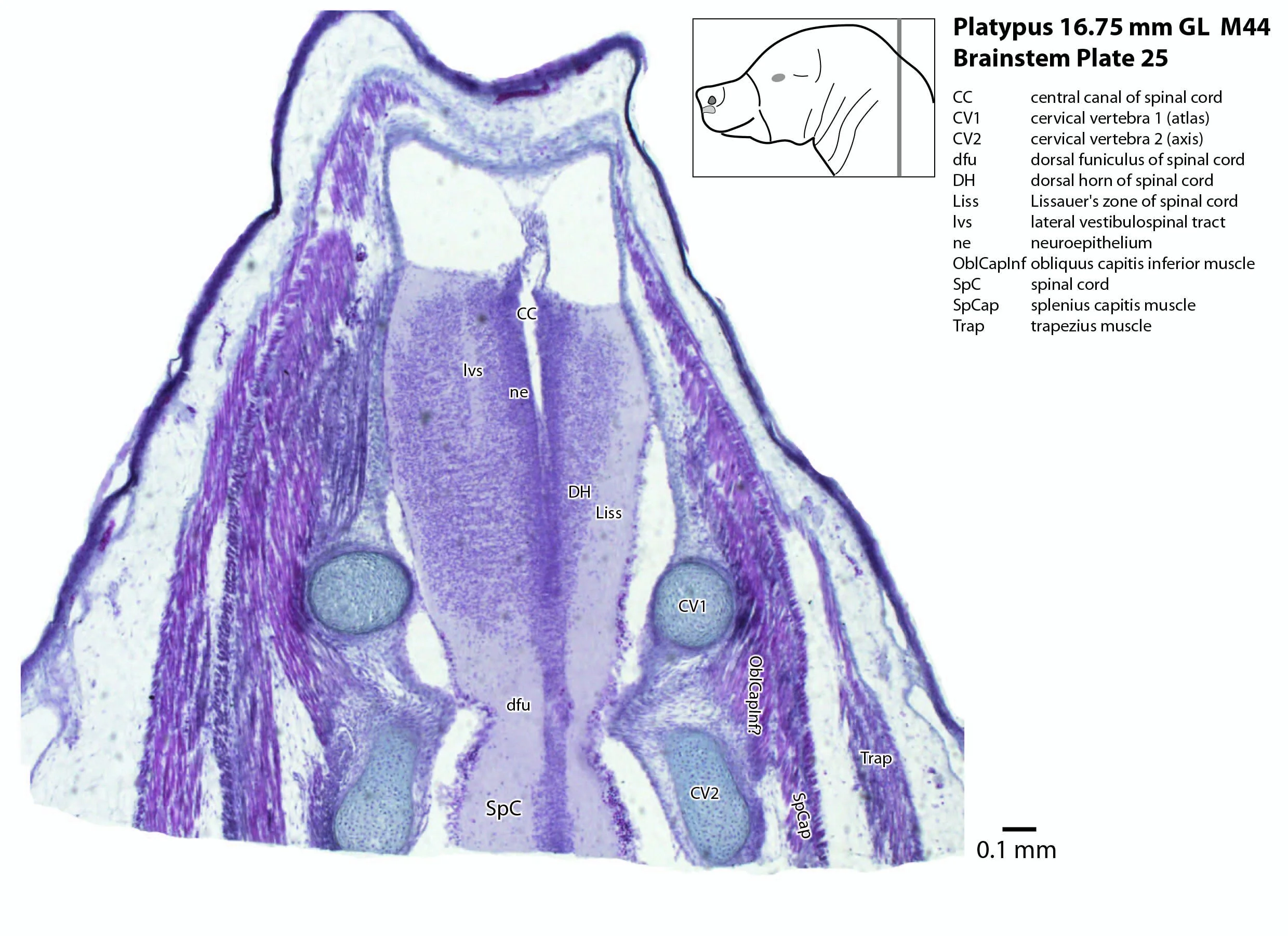
The spinal cord has extensive white matter funiculi (e.g. lfu in plates 18 to 24, vfu in plates 17 and 18) for ascending and descending pathways between the brainstem and spinal cord, but the grey matter is still poorly differentiated.
Acknowledgements
I would like to thank Dr Peter Giere of the MfN, Berlin Germany, for access to the collection and for all his kind help during the work.
References
Ashwell KW (2013) Embryology and post-hatching development of the monotremes. In KWS Ashwell (Ed.), Neurobiology of Monotremes: Brain Evolution in Our Distant Mammalian Cousins (1st ed., pp. 31-46). CSIRO.
Watson CRR (2012) Hindbrain. In The Mouse Nervous System (Eds C Watson, G Paxinos and L Puelles) pp. 398–423. Elsevier, San Diego.