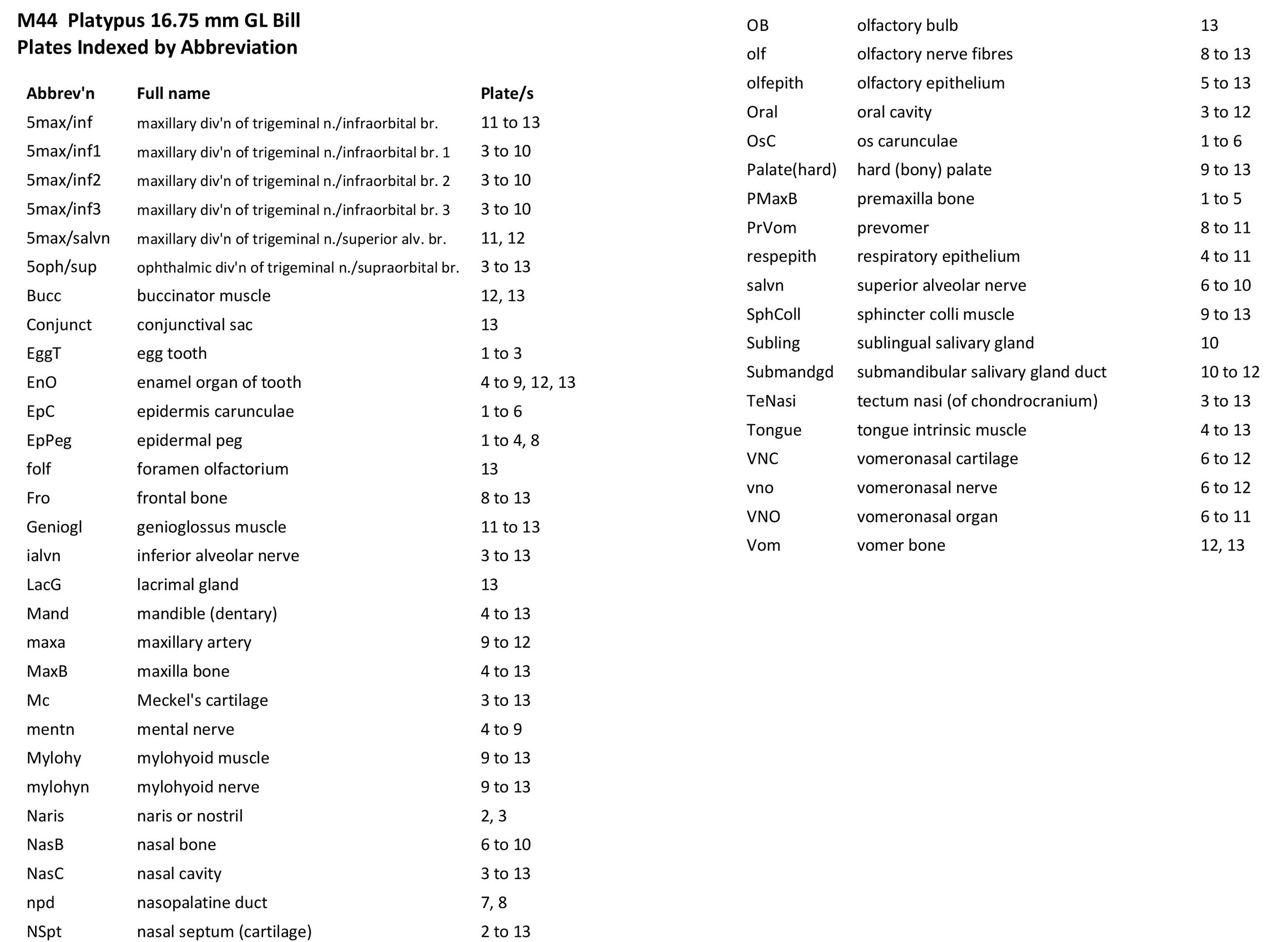
Atlas of the Bill of a Newly Hatched (16.75 mm GL) Platypus (M44)
Introduction
This series of images illustrates two key features of monotreme biology.
1) Caruncle and egg tooth adaptations to break through the leathery egg at hatching.
2) A rich trigeminal nerve sensory supply to the bill/beak for electro- and mechanoreception.
A platypus of 16.75 mm GL (greatest length) would be no more than 1 day after hatching (Ashwell, 2013), where hatching occurs at about 14 to 16 mm GL.
Details on platypus biology can be found at the Platypus page of this website.
Methods
The specimen illustrated here is M44 (a newly hatched platypus) of the Hill collection, stored at the Museum für Naturkunde in Berlin. The most likely site of collection is eastern New South Wales, near Sydney. Note that the specimen card states 16.45 mm GL (newly hatched), but the slides state 16.75 mm GL.
The specimen had been collected in the late 19th century by JP Hill, embedded in paraffin wax and sectioned transversely (frontally) at 10 µm thickness. The stain was not identified on the specimen card, but it appears to be a variant of hematoxylin stain.
Sections at approximately 50 µm intervals were photographed with the aid of a Zeiss Axioplan2 fitted with an AxioCamMRc5 camera. Sections 1 to 146 inclusive were photographed with a 2.5x objective, whereas sections 151 to 176 were photographed with a 1.25x objective. All images were calibrated by photographing a scale bar at the same magnification and components of sections 11 to 146 were photomerged with Adobe Photoshop 2021. Images were placed in Adobe Illustrator 2021 and delineated.
General observations
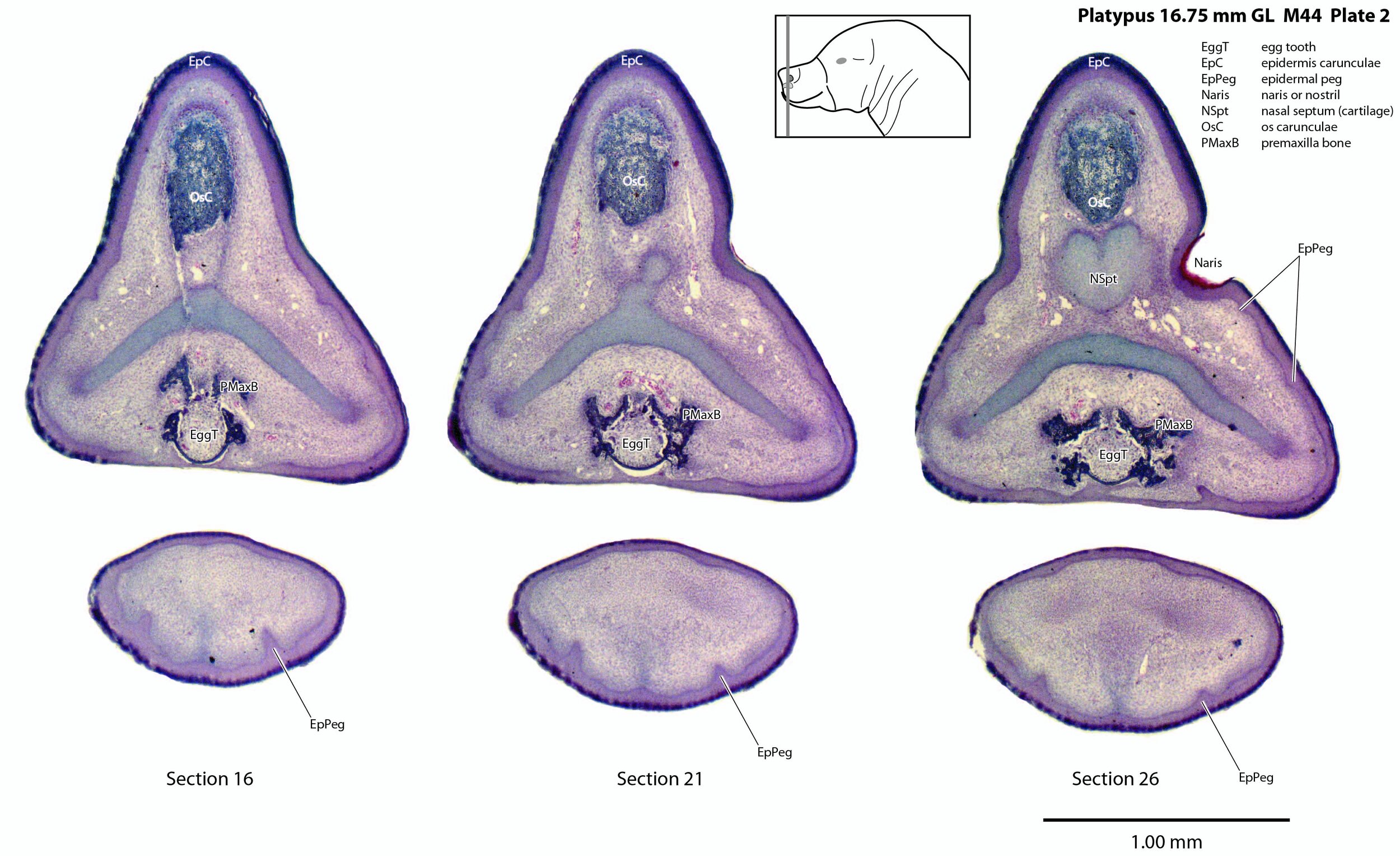
Unlike the horizontally flattened bill of the adult platypus, the bill or beak of this newborn is laterally compressed with a prominent epidermal horn (epidermis carunculae) and egg tooth. The internal structure of the bill is dominated by the chondrocranium (cartilaginous cranium) which encloses the nasal cavity and forms a trough in which the brain sits. There is only limited ossification in the premaxilla, maxilla and mandible (dentary).
Adaptations for hatching from an egg: egg tooth and caruncle
Monotremes have two structures on the rostral (front) end of the snout to rupture the egg membranes at hatching. Repeated flexion/extension of the head on the upper cervical vertebral column will tear the leathery egg membranes. On the dorsum of the snout is a cornified protrusion (the caruncle) which has a bony core (os carunculae – OsC in plate 3) overlain by a thickened zone of epithelium (epidermis carunculae – EpC in plate 3). The ventral extension of the os carunculae has the ventrally directed egg tooth (EggT in plate 2) embedded within it, such that the caruncle and egg-tooth are a single functional complex (e.g. see section 11 of plate 1) that will tear the egg membranes during repeated flexion/extension. The bone of the os carunculae/egg tooth complex is wedged in the newly ossified wings of the premaxilla (PMaxB – sections 31 to 41 of plate 3) and flanked by nasal cartilages. A very similar structure is seen in the newly hatched short-baked echidna (Fenelon et al., 2021).
Rich trigeminal nerve supply of the bill
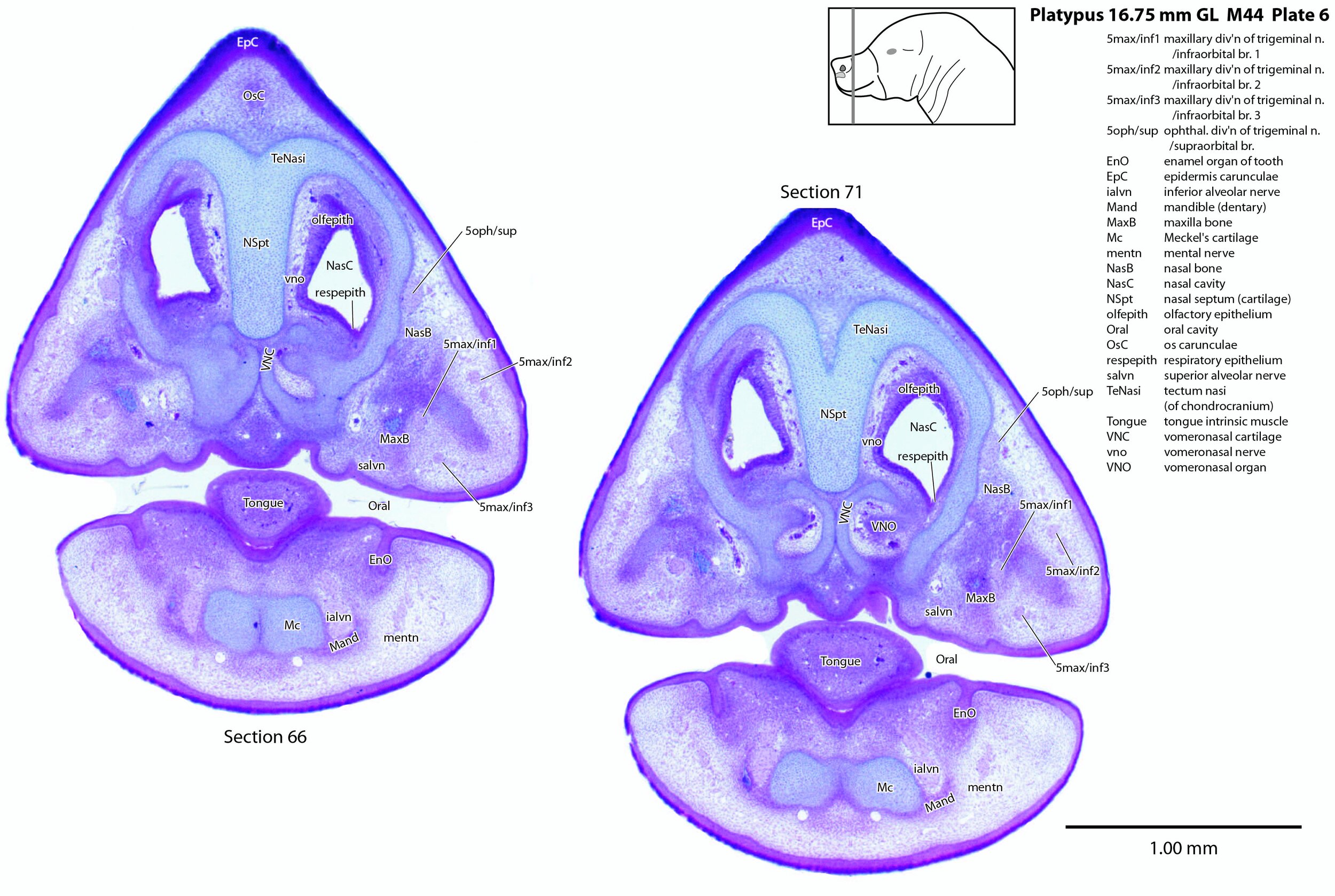
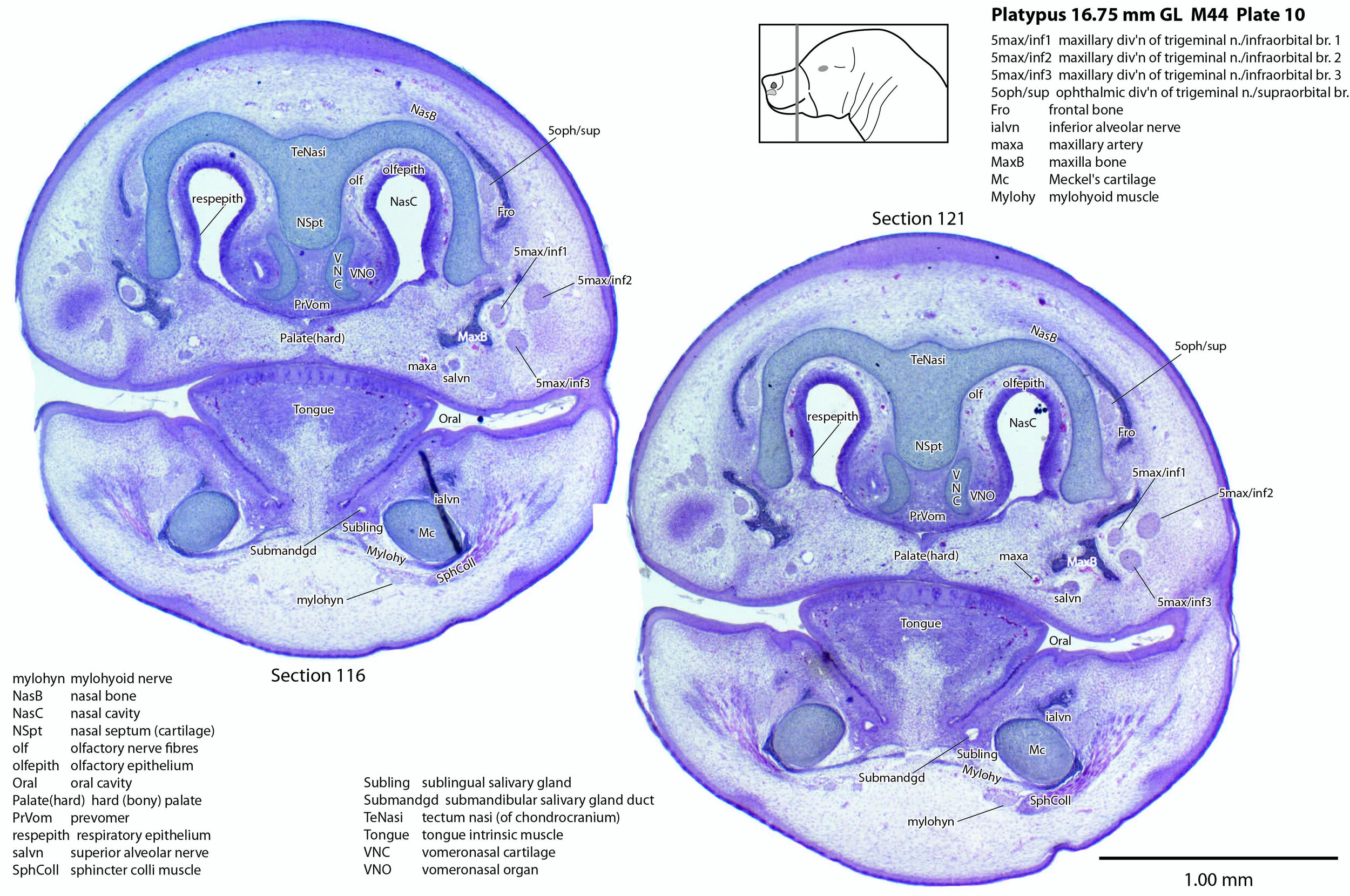
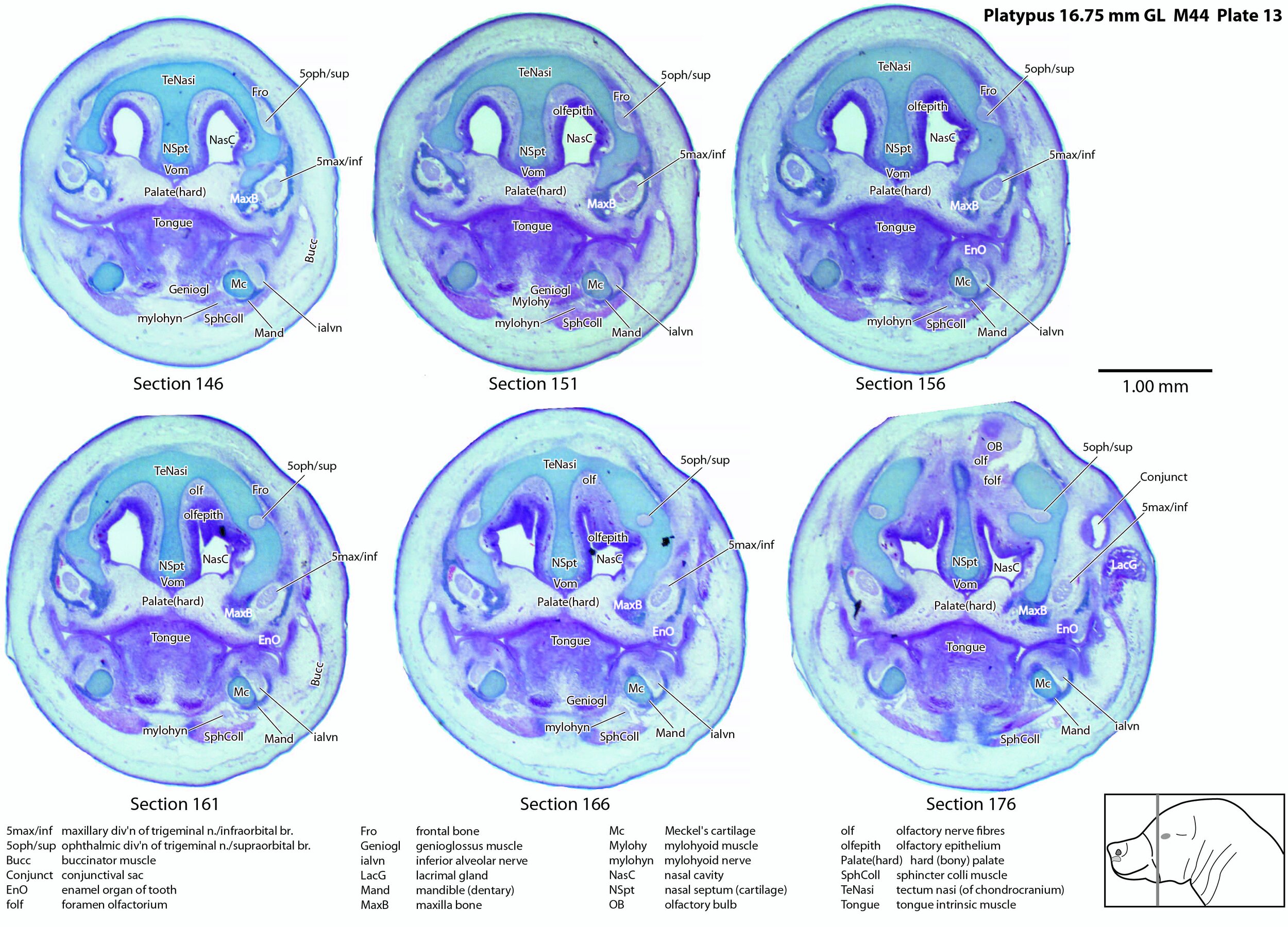
Large branches of all three divisions of the trigeminal nerve can be seen, suggesting that the sensory supply of the bill is rich, even at hatching.
A supraorbital branch of the ophthalmic division (5oph/sup – plates 3 to 13) passes through a tunnel within the chondrocranium (sections 161 to 176, plate 13) before emerging to course between the chondrocranium and frontal and nasal appositional bones. It will supply the proximal dorsum of the bill in adult life.
The infraorbital branch of the maxillary division of the trigeminal nerve is very large and housed in a large tunnel within the maxilla. Three large and distinct superficial sensory branches (5max/inf 1, 2 and 3; plates 3 to 10) will supply the bulk of the dorsum of the upper bill. There is also a large alveolar branch (salvn – plates 6 to 10) supplying the oral side of the upper bill.
At the section levels shown here, the mandibular division of the trigeminal nerve is represented by a large inferior alveolar nerve (ialvn – plates 3 to 13) passing through the developing mandible (dentary). It gives of a large lateral branch (tentatively called the mental nerve (mentn – plates 4 to 9). Collectively these nerves will supply the lower bill (oral and ventral surfaces, respectively). Another nerve, tentatively identified as the mylohyoid nerve (mylohyn – plates 9 to 13) based on its position, was also seen. In other species this would be a predominantly motor nerve to the mylohyoid muscle (Mylohy – plates 9 to 13) and anterior belly of digastric muscle, but given the very small size of those muscles in this species, it is likely that the nerve is predominantly sensory to the ventrum of the lower bill.
Olfactory apparatus
The olfactory epithelium is extensive (olfepith – plates 5 to 13) and olfactory nerve fibres are visible (CN1, olf – plates 8 to 13). A vomeronasal organ VNO – plates 6 to 11) embedded within the vomeronasal cartilage (VNC – plates 6 to 12) can also be seen, and a vomeronasal nerve (vno – plates 6 to 12) traced towards the cranial cavity.
Electrosensory organs
Epidermal pegs and ridges (EpPeg – plates 1 to 4, 8) can be seen along the surface of the bill. These are probably the precursors of the electrosensory glandular apparatus, but are too immature to be functional.
Musculoskeletal system
Most of the skull is a cartilaginous model (chondrocranium) at this stage, with some neuro- and viscerocranial bones (e.g. nasal, frontal bones) forming within membranes around the cartilage.
Nomenclature for musculature is mainly based on Griffiths (1978). Very few muscles are visible at this level and age, apart from the intrinsic lingual muscles (Tongue – plates 4 to 13). A slender buccinator is visible in the rostral cheek (plates 12, 13), as well as a putative sphincter colli (SphColl – plates 9 to 13).
Acknowledgements
I would like to thank Dr Peter Giere of the MfN, Berlin Germany, for access to the collection and for all his kind help during the work.
References
Ashwell KW (2013) Embryology and post-hatching development of the monotremes. In KWS Ashwell (Ed.), Neurobiology of Monotremes: Brain Evolution in Our Distant Mammalian Cousins (1st ed., pp. 31-46). CSIRO.
Fenelon JC, Bennetts A, Anthwal N, Pyne M, Johnston SD, Evans AR, Tucker AS, Renfree MB (2021) Getting out of a mammalian egg: the egg tooth and caruncle of the echidna. Dev Biol doi: 10.1016/j.ydbio.2022.12.005.
Griffiths M (1978) The Biology of the Monotremes. Academic, New York.