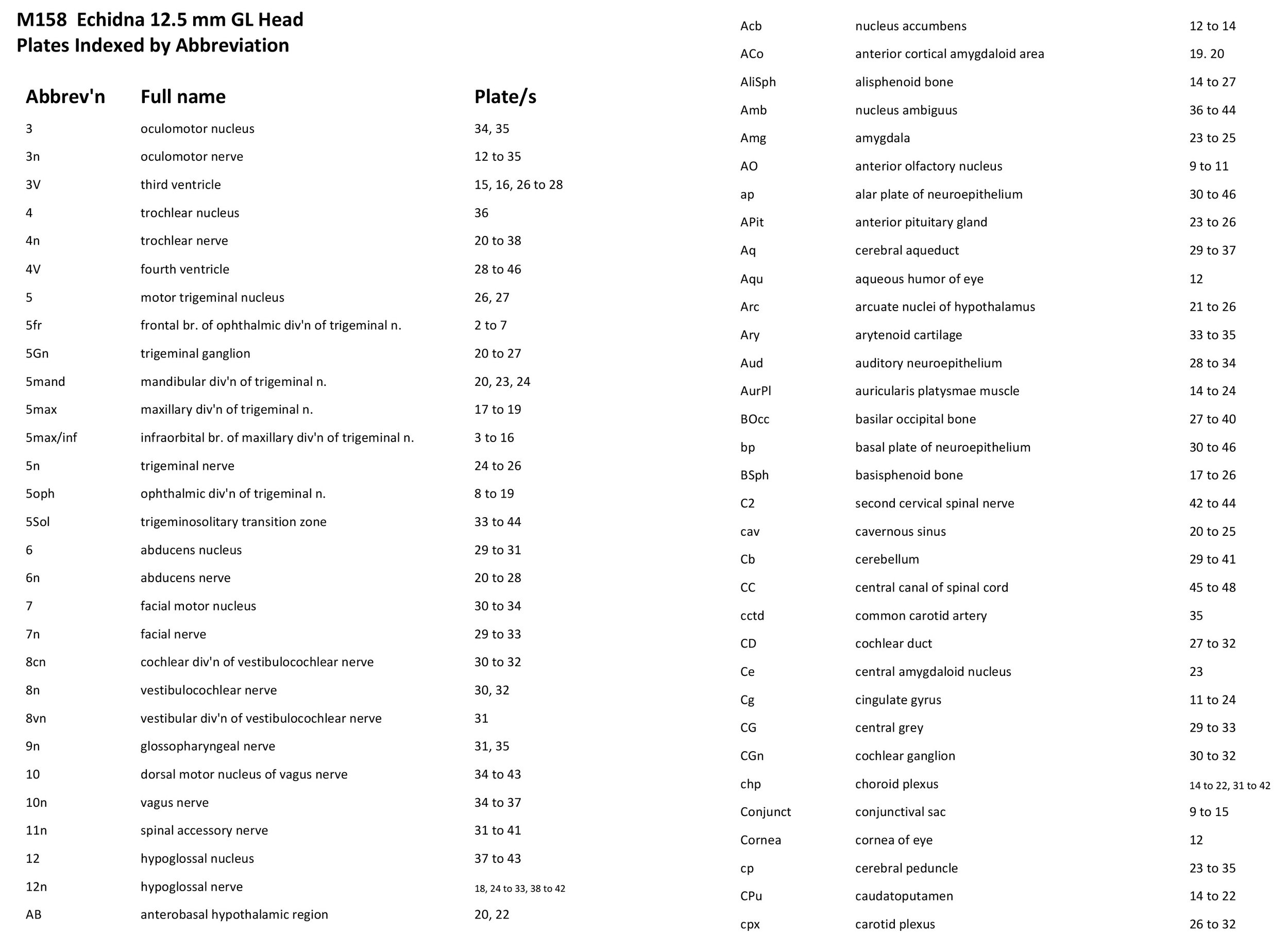
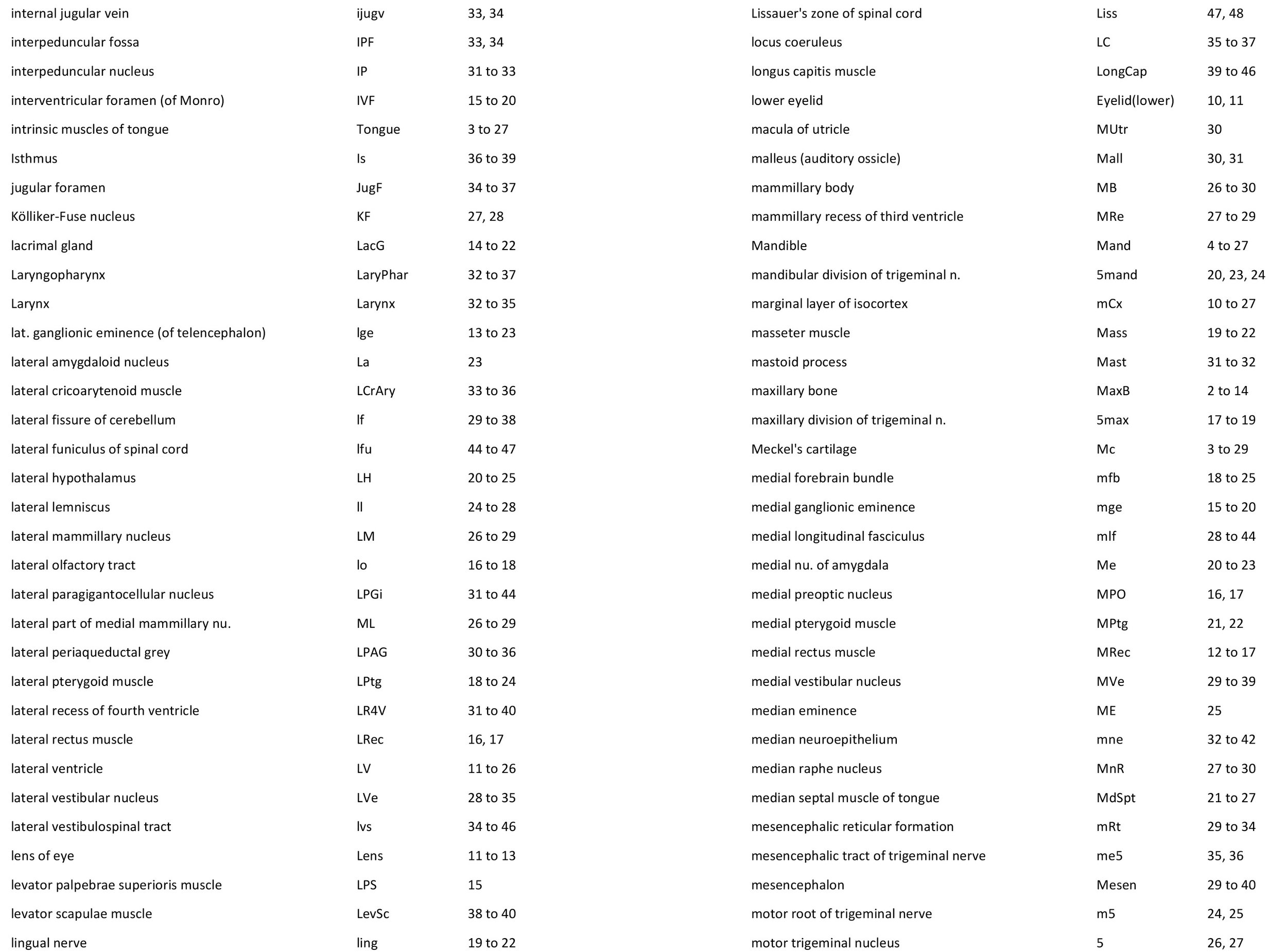
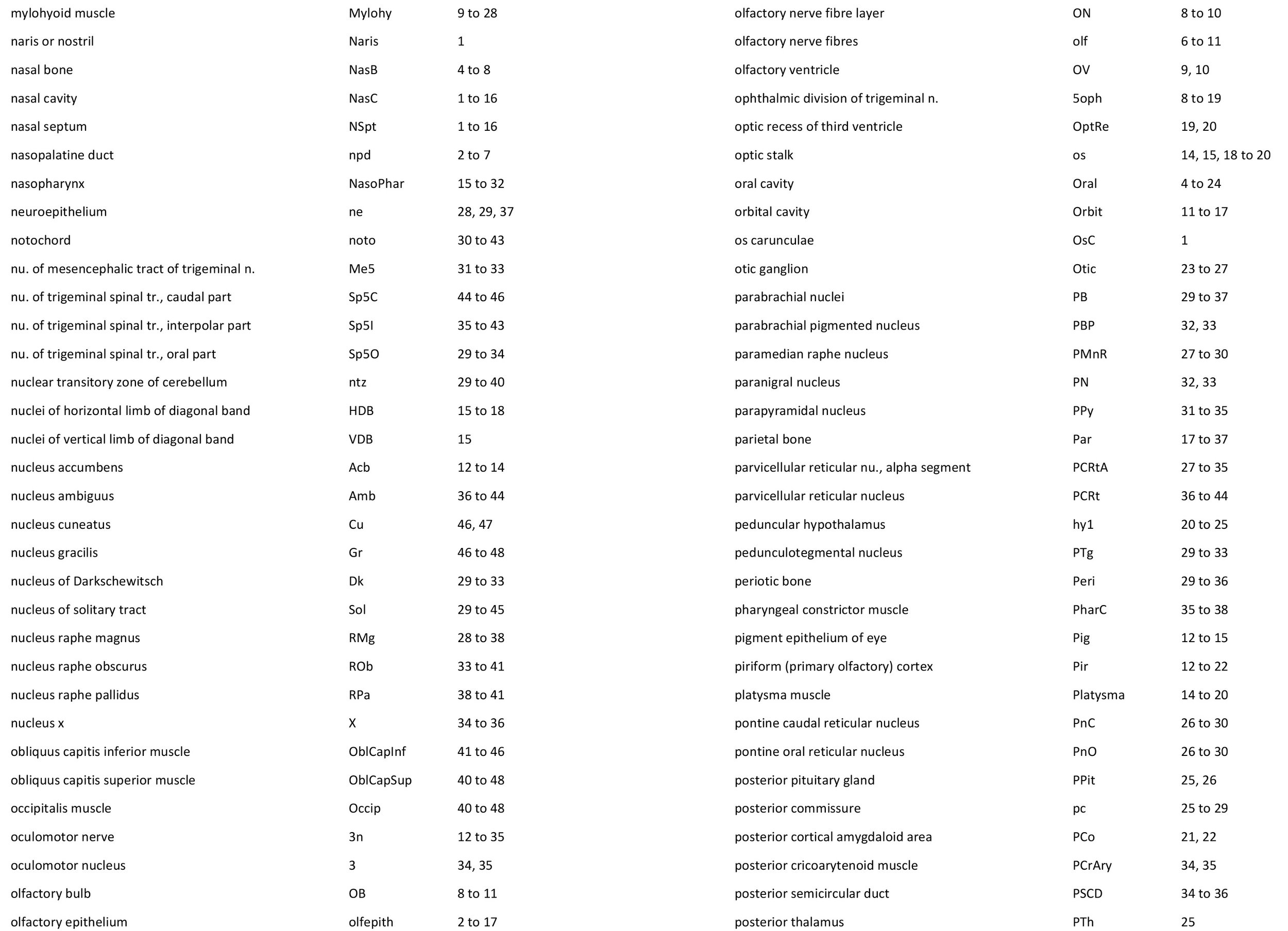
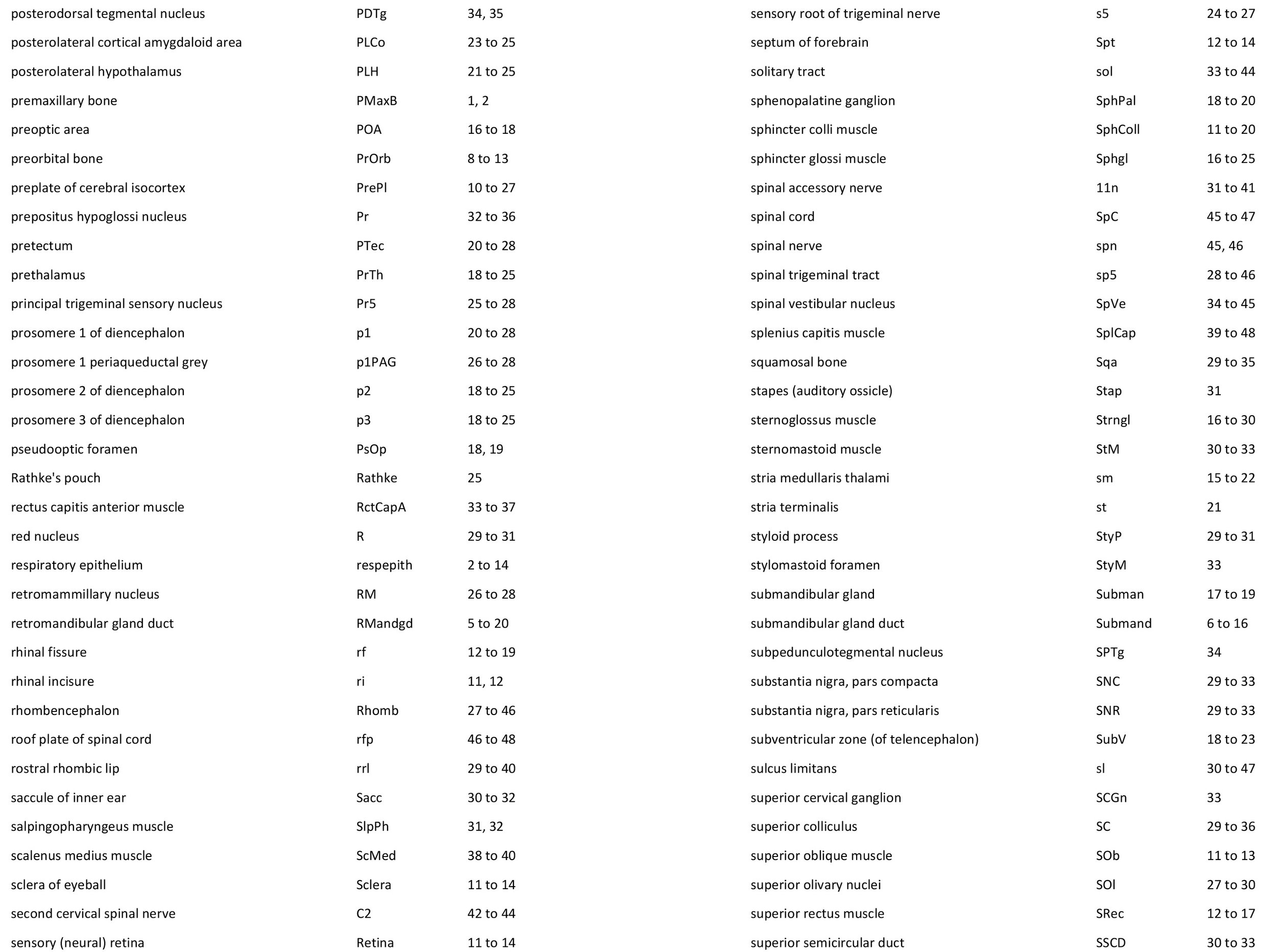
Atlas of the Head of a 12.5 mm GL (Prehatching) Echidna (M158)
Introduction
The short-beaked echidna specimen depicted here was labelled as “Echidna GL 12.5 mm”, where GL refers to greatest length. The subspecies is probably Tachyglossus aculeatus aculeatus, given that the most likely point of collection is eastern New South Wales near Sydney. An echidna in ovo of 12.5 mm GL is probably 2 days before hatching (Ashwell, 2013)(where hatching occurs at about 14 to 16 mm GL) and the specimen would be stage 22 (Werneburg and Sánchez-Villagra, 2011). The high quality of the sectioning and staining and the importance of the age (immediately before hatching) make this a significant specimen.
Details on short-beaked echidna biology can be found at the Short-beaked Echidna page of this website.
Methods
The incubation (in ovo) stage specimen illustrated here is M158 of the Hill collection stored at the Museum für Naturkunde in Berlin. The specimen had been collected in the late 19th century by JP Hill, embedded in paraffin wax and sectioned at 10 µm thickness. The stain was not identified on the specimen card but appears to be haematoxylin and eosin.
Images in plates 1 to 9, and 29 to 48, were photographed by Ken Ashwell in Berlin in 2022. The sections at approximately 80 µm intervals were photographed with the aid of a Zeiss Axioplan2 fitted with an AxioCamMRc5 camera. All images were calibrated by photographing a scale bar at the same magnification and photomerged with Adobe Photoshop 2021. Merged images were placed in Adobe Illustrator 2023 and delineated.
The sections between plates 9 and 29 were not available in 2022, so images previously taken of those sections by KK Sulik (please see the MfN for access) have been used instead. Please note that those images are of a substantially lower resolution. Those images were also placed in Adobe Illustrator 2023 and delineated.
Notes on the specimen
General observations
The gut is still in its physiological herniation. The beak has early electroreceptors (as epidermal pegs) on the underside of the snout in a single row on each side of the egg tooth (plate 1). Spines have begun to develop as epidermal papillae, but these have yet to reach the panniculus carnosus layer.
Nasal cavity and olfactory apparatus
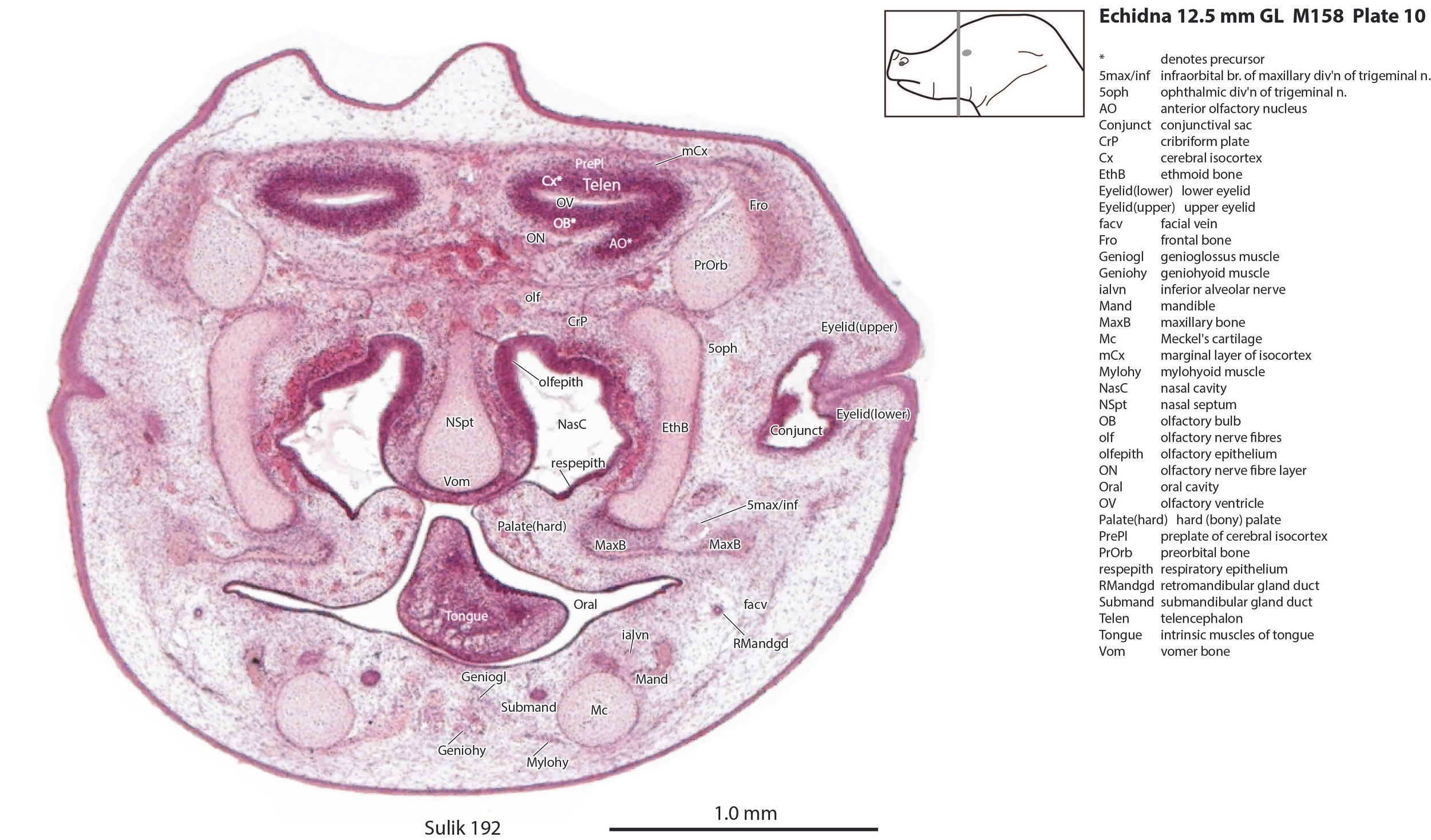
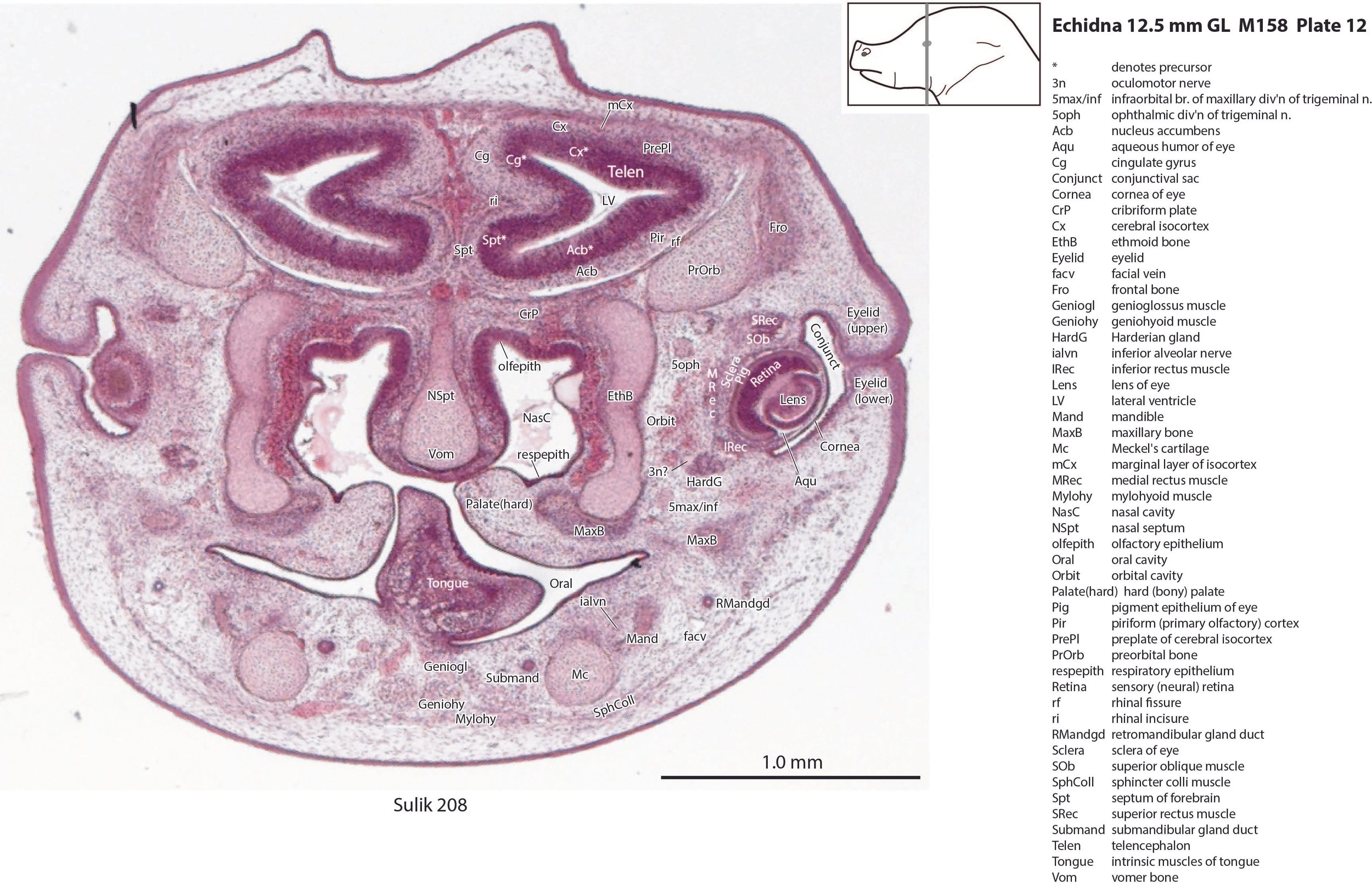
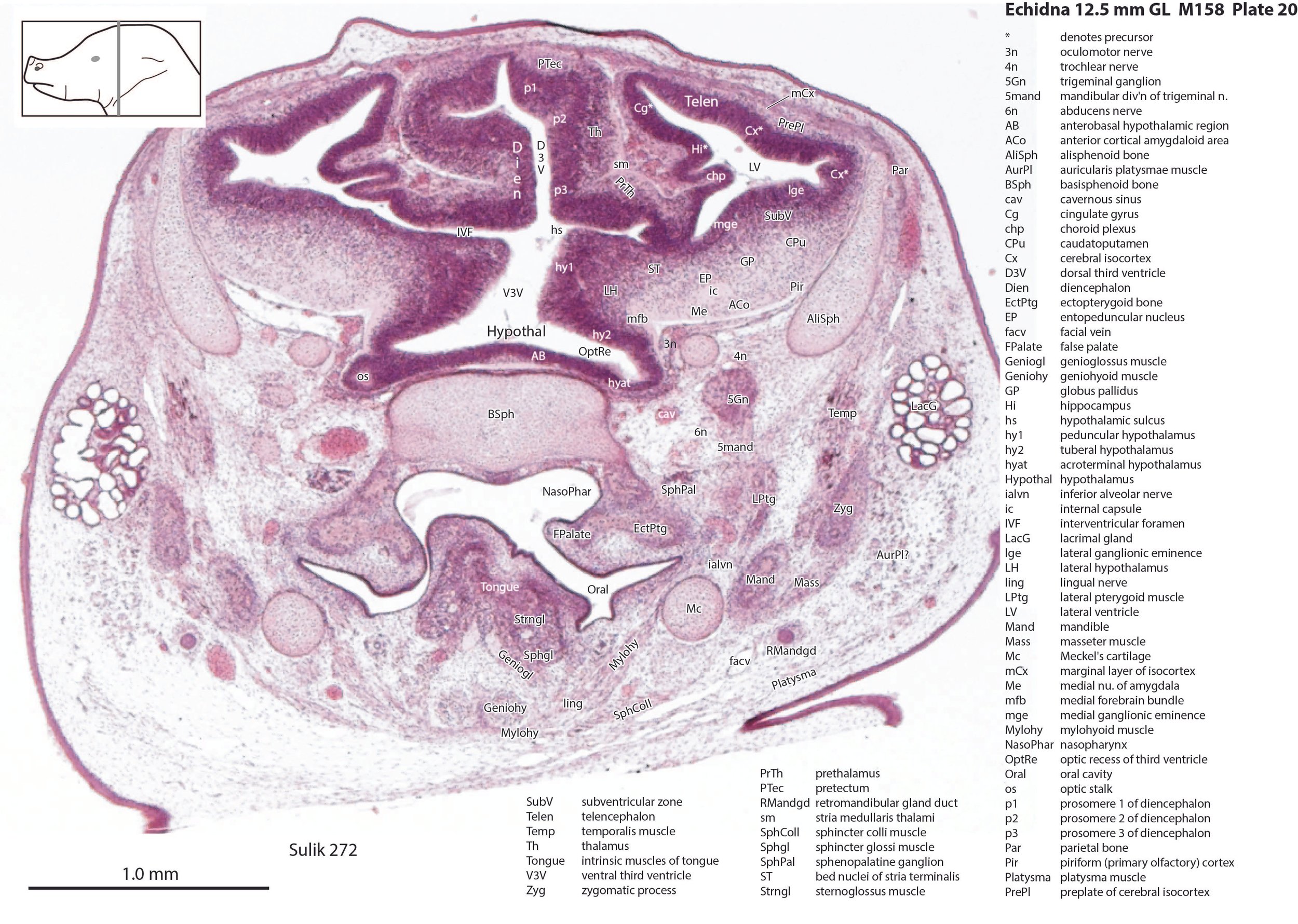
The nasal cavity (plates 1 to 16) shows the beginnings of complex folding of its lateral wall. Olfactory nerve fibres (olf in plates 6 to 11) are leaving the nasal cavity and penetrating the olfactory bulb (OB in plates 8 to 11). The olfactory bulb has a thick proliferative zone and a relatively thin postmitotic region with the suggestion of some large cells (perhaps early output neurons) but only a rudimentary lateral olfactory tract (lo in plates 16 to 18). There is a prominent cleft (rhinal incisure – ri in plates 11, 12) between the OB and the rest of the developing telencephalon. The putative piriform (primary olfactory) cortex is more caudal (plates 12 to 22) but poorly differentiated.
Cerebral isocortex and hippocampus
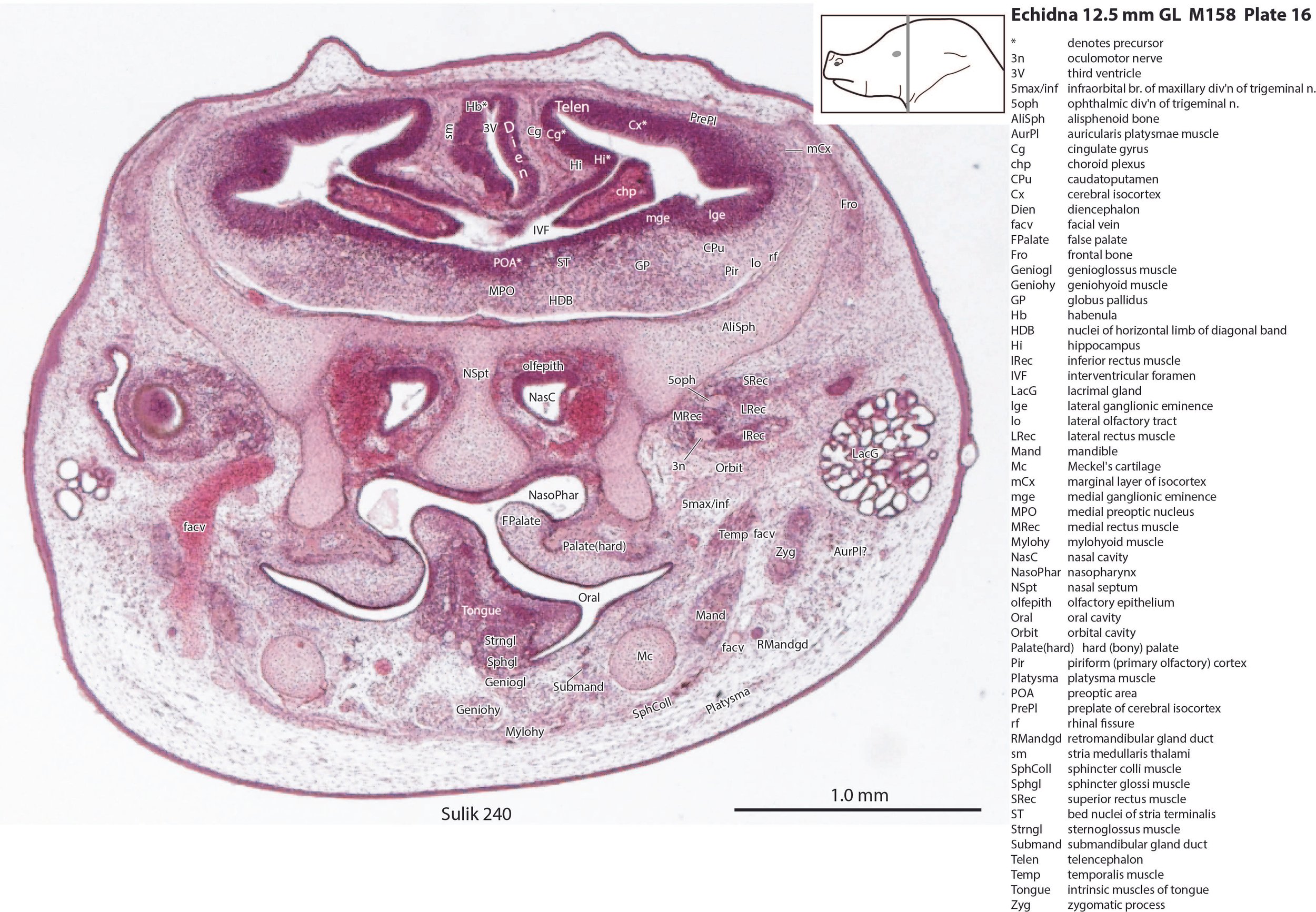
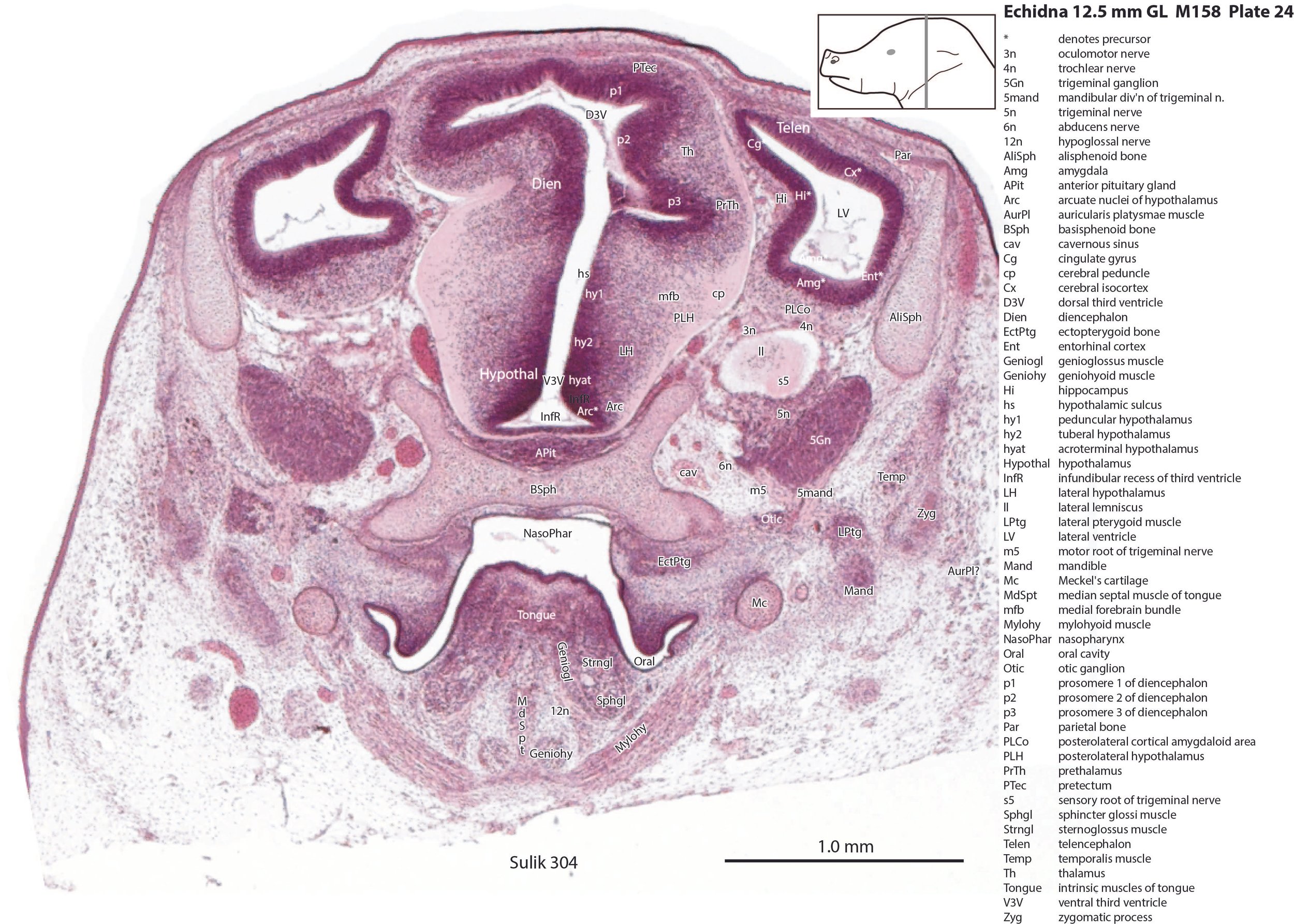
The pallium has many points of flexure which may be precursors of the adult pattern of gyrification. There is no obvious cortical plate, but the preplate region (PrePl in plates 10 to 27) is populated by scattered postmitotic neurons. The nascent hippocampal formation (Hi in plates 13 to 25) has begun to infold but is still quite rudimentary, with neither cornu Ammonis or dentate gyrus. There are large postmitotic cells along the medial wall of the frontal pole of the telencephalic vesicle, which may be early cingulate gyrus.
Striatum, pallidum and amygdala
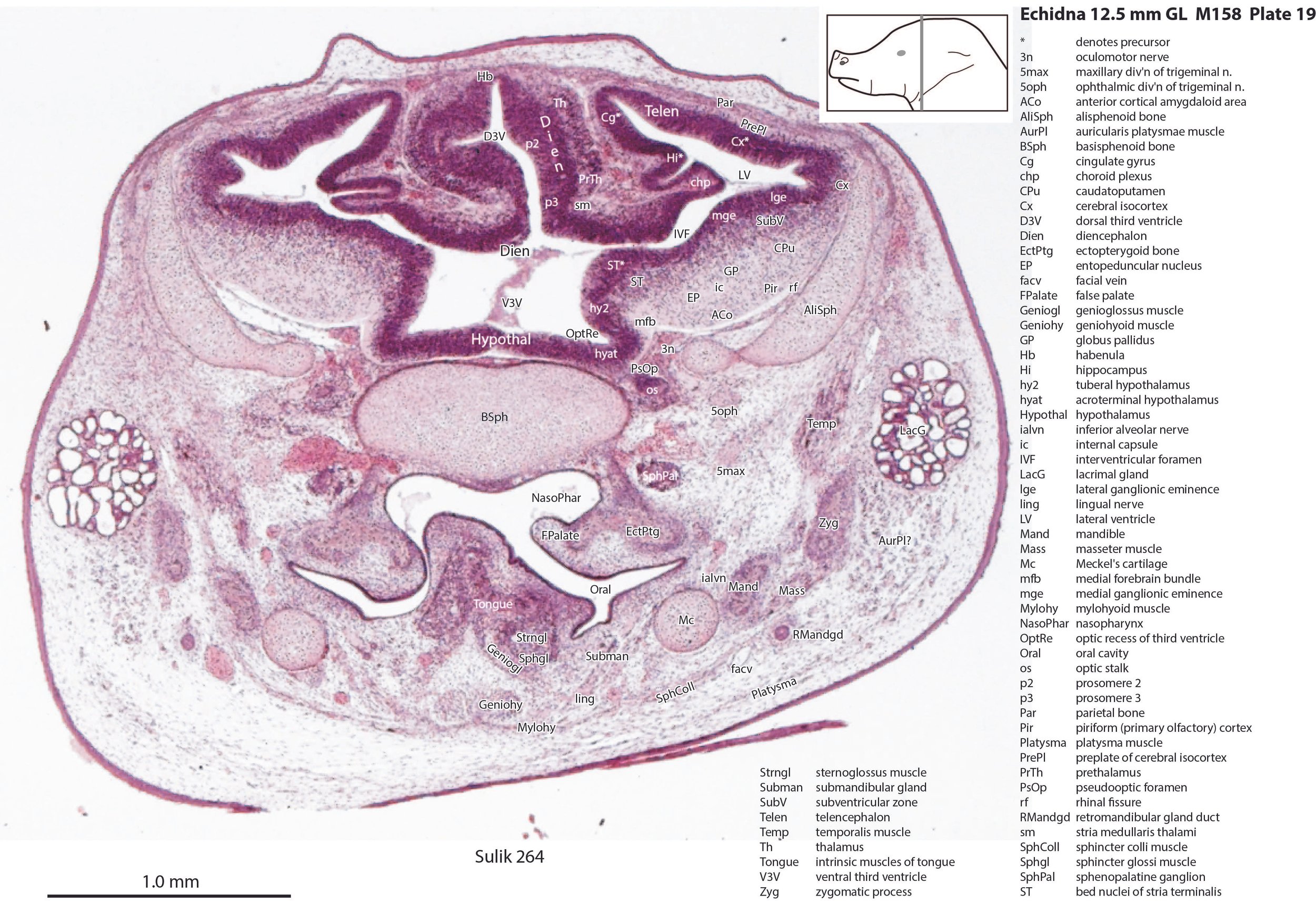
The striatum, pallidum and amygdala are rudimentary, but neurogenesis for the striatum and cortex is underway, with lateral and medial ganglionic eminences prominent (lge in plates 13 to 23, mge in plates 15 to 20), as well as an underlying subventricular zone (SubV in plates 18 to 23). A fibre bundle which may be the internal capsule (ic in plates 19 to 22) begins in the centre of the developing pallidostriatal region and can be traced around the developing entopeduncular nucleus to the thalamus.
Hypothalamus
The traditional view has been that the hypothalamus is a derivative of the diencephalic brain vesicle, but gene-mapping studies suggest that the hypothalamus should be considered a derivative of the proneuromere rostral to the p3 and therefore is a discrete part of the forebrain, quite separate from the diencephalon (Puelles et al. 2012).
The hypothalamus proliferative compartments are divided into peduncular (hy1), tuberal (hy2) and acroterminal (hyat) segments (see plates 19 to 25). Some lateral hypothalamic regions have postmitotic neuronal populations (e.g. LH in plates 20 to 25, PLH in plates 21 to 25), but medial hypothalamic nuclei have yet to emerge.
Diencephalon
The diencephalic proliferative compartments are divided into three prosomeres (p1 – pretectum in plates 20 to 28, p2 – thalamus proper in plates 18 to 25, and p3 – prethalamus in plates 18 to 25). There is still a very thick proliferative zone in all three prosomeres, but some differentiation of nuclei is beginning to take place in the lateral thalamus.
Midbrain and isthmus
The eye muscle nuclei are identifiable (3 – oculomotor in the mesencephalic segment, see plates 34, 35; 4 – trochlear nucleus in the isthmic segment, see plate 36), but nuclear boundaries are difficult to distinguish in the midbrain and isthmic tegmentum. The substantia nigra is visible in plates 29 to 33, alongside an early cerebral peduncle (cp in plates 23 to 35), but most other midbrain nuclei are undifferentiated.
Cerebellum
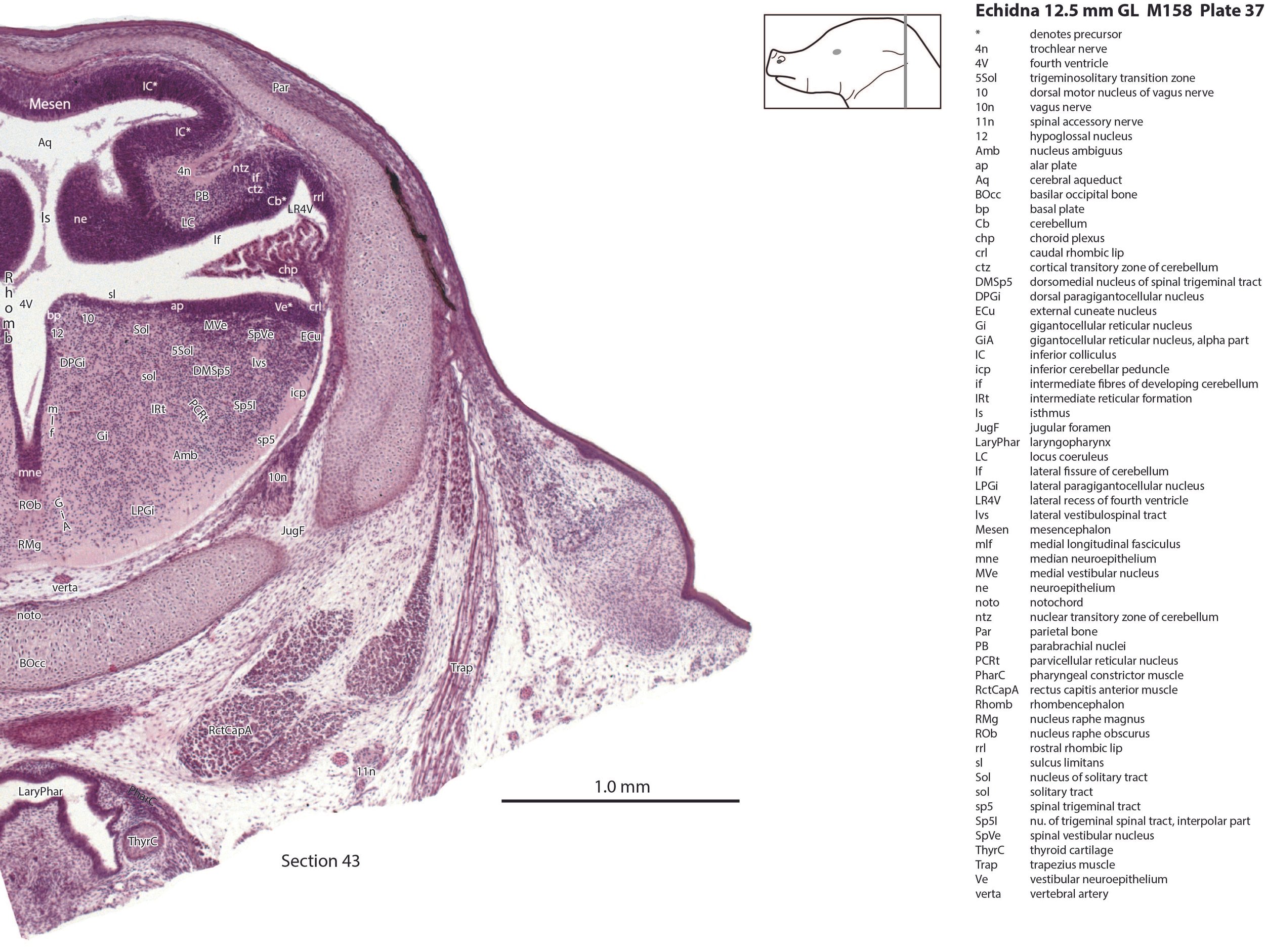
The nuclear transitory zone (ntz) and cortical transitory zone (ctz) in plates 29 to 40 both appear to be present, but the external granular layer has not yet begun migration from the germinal trigone (rostral rhombic lip – rrl in plates 29 to 40). Fibres which may be the hook bundle of Russell (hbR in plate 40) and the inferior cerebellar peduncle (icp in plates 27 to 45) can be seen, but no other evidence of significant afferent or efferent fibres.
General brainstem
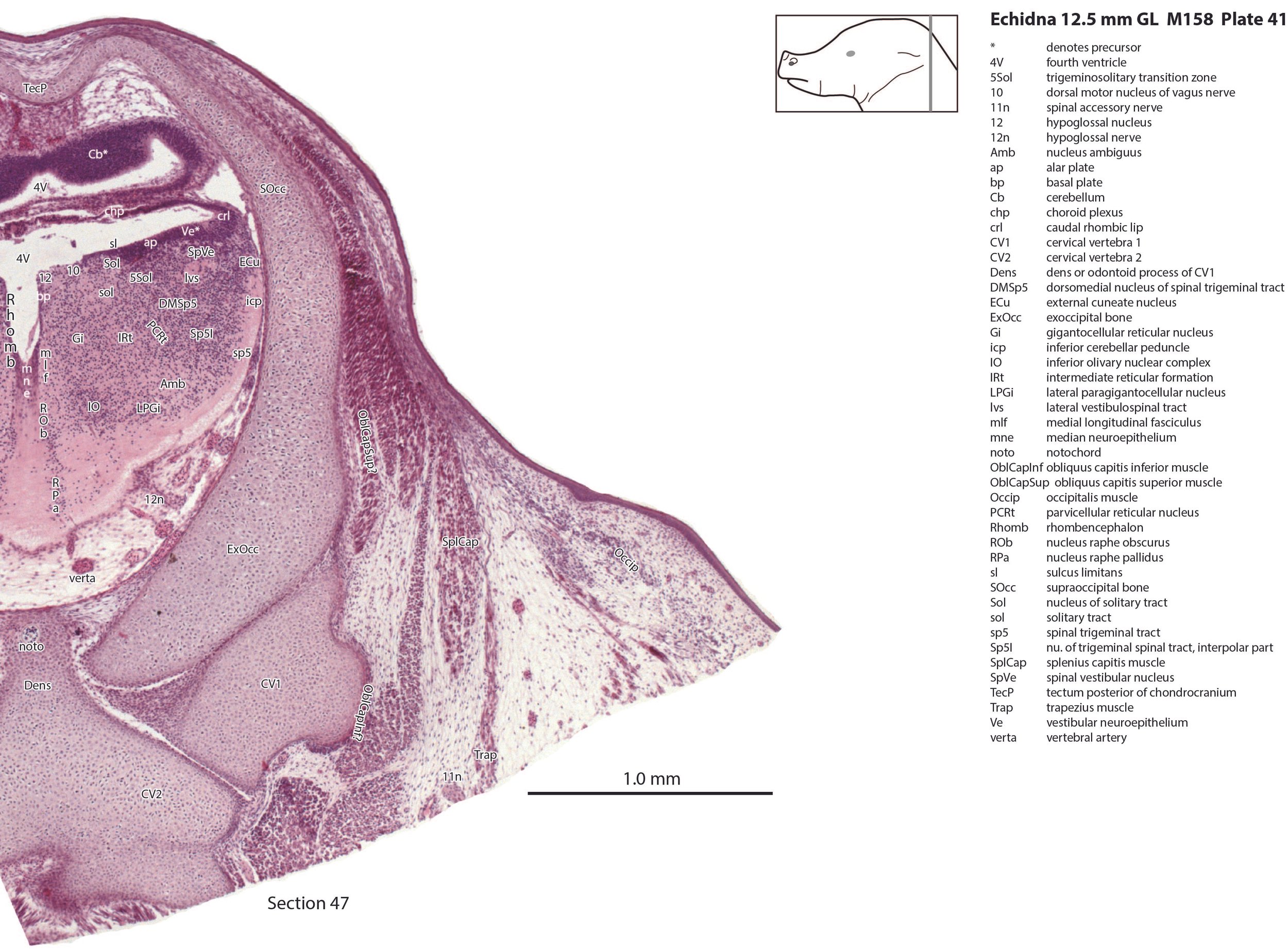
There is a differentiation into medial magnocellular (Gi, GiV, LPGi) and lateral parvicellular (PCRt, PCRtA) nuclei and the raphe nuclei (DR, MnR, RMg) are emerging. The medial magnocellular nuclei presumably give rise to bulbospinal pathways. Some neurons have begun to settle into the inferior olivary nuclear complex (IO in plates 40 to 42) indicating that migration from the caudal rhombic lip is underway.
Trigeminal system
The trigeminal ganglion (5Gn in plates 20 to 27) is clearly smaller than in the platypus of a comparable size. The principal trigeminal nucleus (Pr5 in plates 25 to 28) is simpler than in the platypus of a similar age, with a less prominent granule cell layer, but a small region is in continuity with the rrl.
Vestibular system
The vestibular nuclei are poorly differentiated, but the semicircular canals and all other parts of the otocyst are quite elaborated. Maculae are developing in the utricle and saccule (see macula of the utricle - MUtr in plate 30).
Eye
The lens is rudimentary (plates 11 to 13), as is the sensory retina (plates 11 to 14). An optic stalk can be identified (os in plates 14, 15 and 18 to 20) but no optic nerve, and the central visual centres like the superior colliculus (SC in plates 29 to 36) are still poorly differentiated.
Musculoskeletal system
Most of the skull is a cartilaginous model (chondrocranium) at this stage, with some neuro- and viscerocranial bones forming within membranes around the cartilage.
Rather than use transient chondrocranial nomenclature, labels for the adult bone that would develop from the cartilage model have been assigned. The two exceptions to this are the tectum nasi (TeNasi – plates 1 to 7) and tectum posterius (TecP – plates 38 to 42), both midline roof features of the chondrocranium (Griffiths, 1978).
Nomenclature for musculature is mainly based on Griffiths (1978). The two most striking muscle groups are the superficial (subdermal) facial group and the lingual musculature.
The superficial facial group includes a (pre)auricularis platysmae (AurPl – plates 14 to 24), and an occipitalis muscle (Occip – plates 40 to 48). Presumably these muscles are important for shifting the spines forward to protect the head during the defensive posture.
The lingual muscles include the sternoglossus (Strngl – plates 16 to 30) and median septal (MdSpt – plates 21 to 27) muscles (both described by Griffiths, 1978), the first of which must be a powerful lingual retractor, a genioglossus (Geniogl – plates 8 to 24) presumably a protrusor of the tongue, as well as a muscle which spirals obliquely around the distal sternoglossus, and which has been called here sphincter glossi (Sphgl – plates 16 to 25) because it would be able to compress the sternoglossal belly and cause protrusion. A small hyoglossus (Hyogl – plates 29, 30), presumably a lingual retractor and depressor, was also found.
The masticatory muscles are relatively small in volume, especially the masseter (Mass – plates 19 to 22) and temporalis (Temp – plates 16 to 24), consistent with the limited load requirement for crushing of food. Some protraction and lateral movement of the mandible (dentary) would be needed for crushing arthropods, and these are provided by the lateral and medial pterygoid muscles (LPtg – plates 18 to 24; MPtg – plates 21, 22).
Acknowledgements
I would like to thank Dr Peter Giere of the MfN, Berlin Germany, for access to the collection and for all his kind help during the work.
References
Ashwell KW (2013) Embryology and post-hatching development of the monotremes. In KWS Ashwell (Ed.), Neurobiology of Monotremes: Brain Evolution in Our Distant Mammalian Cousins (1st ed., pp. 31-46). CSIRO.
Griffiths M (1978) The Biology of the Monotremes. Academic, New York.
Puelles L, Martinez-de-la-Torre M, Bardet S and Rubenstein JLR (2012a) Hypothalamus. In The Mouse Nervous System (Eds C Watson, G Paxinos and L Puelles) pp. 221–312. Elsevier, San Diego.
Werneburg I and Sánchez-Villagra MR (2011) The early development of the echidna, Tachyglossus aculeatus(Mammalia: Monotremata), and patterns of mammalian development. Acta Zoologica (Stockholm) 92, 75–88.