Synaptophysin Immunoreactivity in the Head and Neck of the P0 (Newborn) Tammar Wallaby (Notamacropus eugenii)
Introduction
The tammar wallaby (Notamacropus eugenii) is a small macropod originally found in Western and South Australia and on ten or more offshore islands. Please see the introduction for the P5 H&E atlas for details of tammar wallaby biology.
Synaptophysin (major synaptic vesicle protein p38) is a membrane glycoprotein that is found in presynaptic membranes of axons throughout the nervous system and in neuroendocrine cells. It may be important in the regulation of activity-dependent synapse formation (Tarsa and Goda, 2002), but its precise function is still uncertain. It is used here as a marker for development of axonal connections in the immature tammar nervous system.
Methods
The wallaby depicted in the series of colour plates was obtained from a breeding colony at the Australian National University (ANU) in the Australian Capital Territory (ACT).
All experimental procedures were approved by the Animal Ethics Experimentation Committee of the ANU, conform to NIH principles of laboratory animal care and were carried out according to the ethical guidelines of the National Health and Medical Research Council (Australia). The age of the pouch young animal was determined either directly by noting the elapsed time from the date of birth, which was designated P0, or from measurements of head-length and reference to a chart of head-lengths of animals of known age. This is accurate to within ±2 days.
The pouch-young tammar was anaesthetized by hypothermia and perfused with normal saline followed by Bouin’s fixative. The head was stored in 70% ethanol prior to embedding in paraffin and sectioning coronally at a thickness of 10 µm. Embedding, sectioning and immunohistochemistry all took place in the laboratory of Professor Jürgen Mai at the Heinrich Heine University in Düsseldorf. The sections were mounted on subbed glass slides. After immunostaining, the slides were coverslipped with DePeX.
Each section was photographed with the aid of a Zeiss Axiophot photomicrographic system and a Canon EOS 400Dbody. Images were placed in Adobe Illustrator 2021 and delineated. Developmental regions (i.e. neuroepithelium) destined to give rise to adult structures have been denoted by the adult structure’s name with an asterisk (e.g. Cb* denotes the cerebellar developmental field of the rhombic lip).
Observations
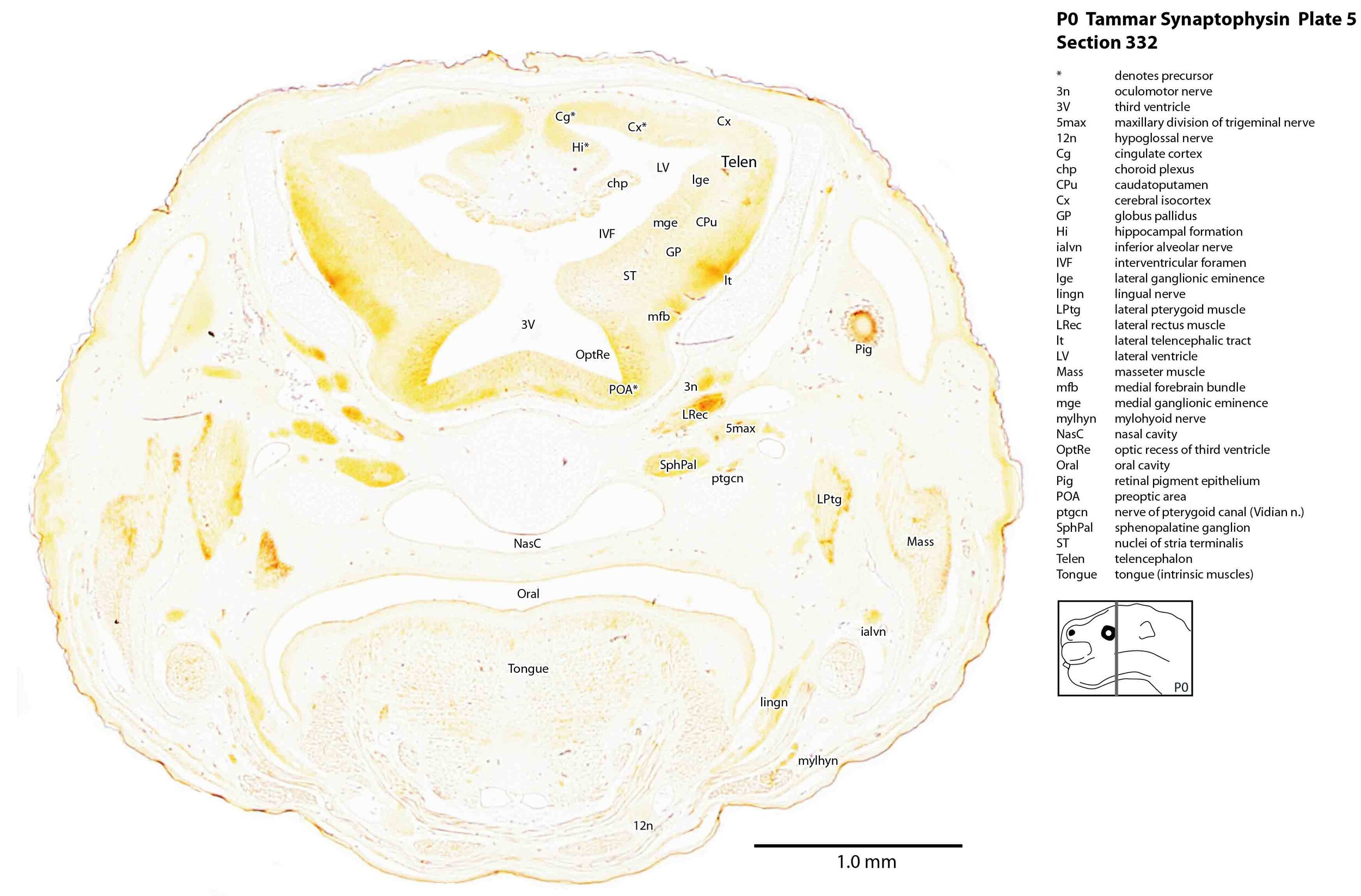
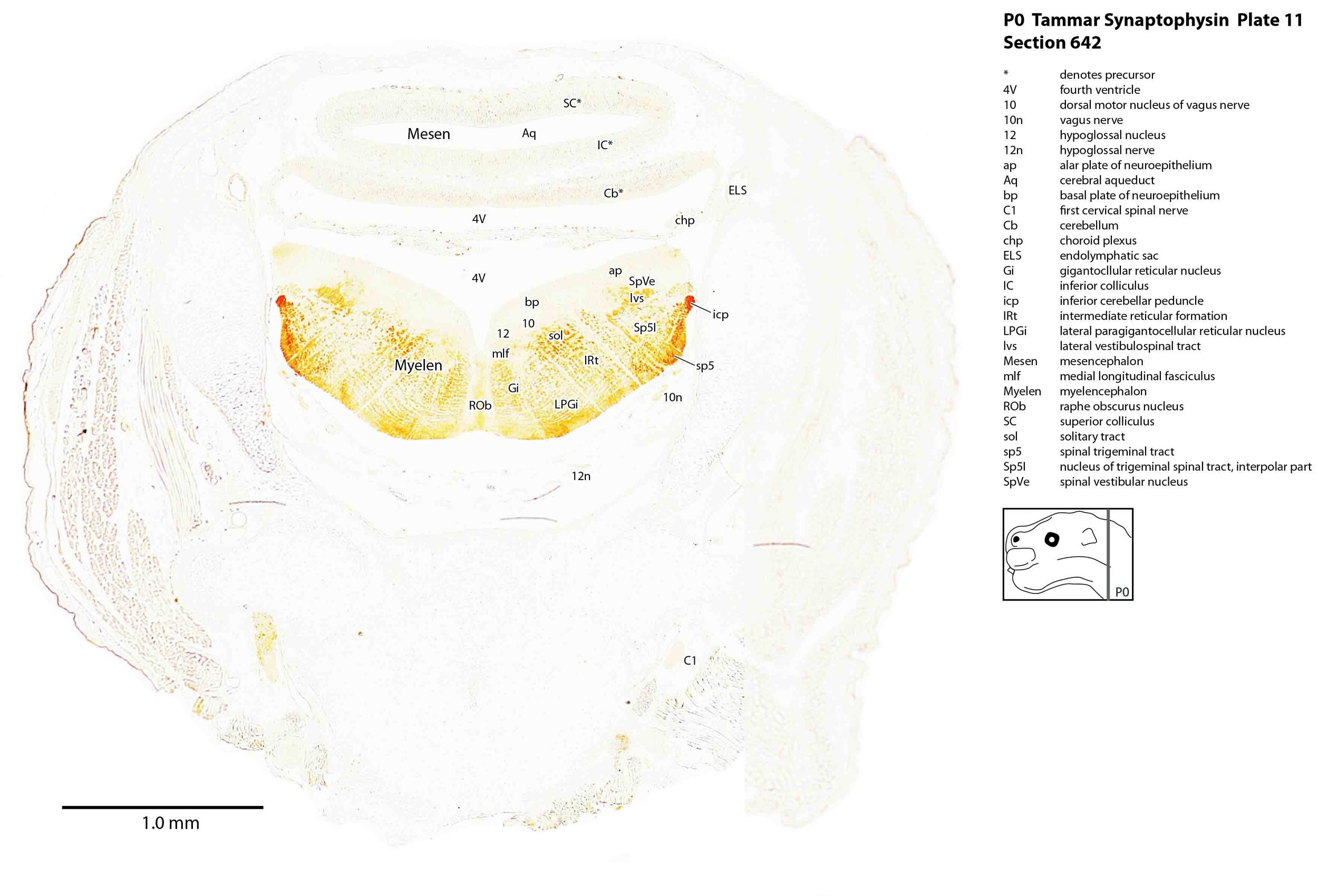
Immunoreactivity for synaptophysin was strongest in components of the trigeminal sensory pathways. These include trigeminal nerve branches such as 5max (plate 5) and 5mand (plates 6, 7), the trigeminal ganglion 5Gn (plates 6, 7), the trigeminal spinal tract sp5 (plates 8 to 12), and the trigeminal sensory nuclei Sp5O (plates 8 to 10) and Sp5I (plates 11, 12). This is consistent with a major role for trigeminal somatosensation in newborn behaviours such as teat location and attachment. Activity was also quite strong in the terminal nerve (cranial nerve 0, plates 1 to 3).
Immunoreactivity was weak in components of the olfactory system (e.g. olfactory epithelium, olfactory nerve fibres and the olfactory bulb – plate 1) and auditory pathways (cochlear ganglion – plate 9). Immunoreactivity was also relatively weak in the cerebral isocortex (Cx – plates 2 to 7), indicating that synapse formation is still very immature at the cortical level.
Some strong activity was seen in forebrain tracts like the medial forebrain bundle (mfb – plates 5 to 7) and lateral telencephalic tract (lt – plates 5 to 7), suggesting that some longitudinal connections within the basal forebrain are forming.
The magnocellular components of the brainstem (Gi – plats 8 to 11, DPGi – plate 10, LPGi – plates 10, 11) also showed strong immunoreactivity, consistent with their role in descending regulation of spinal cord centres mediating early forelimb locomotion. The lateral vestibulospinal tract (lvs – plates 11, 12) also showed immunoreactivity, suggesting a role for descending vestibulospinal connections in behaviour of the newborn tammar.
In the muscular system, there is strong immunoreactivity in synaptic contacts to the extraocular muscles (MRec, SRec, IRec, LRec - Plates 3 to 5).
Please note that there is some natural pigmentation associated with the retinal pigment epithelium (Pig - Plates 2 to 5), which is not synaptophysin immunoreactivity.
References
Tarsa L, Goda Y (2002) Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci 99: 1012-1016.