Atlas of the Head and Neck of a Newborn Bandicoot (MS221)
Introduction
The specimen depicted here was labelled “Perameles obesula” and is likely Isoodon obesulus, the southern brown bandicoot. This species has a distribution along the south-eastern coast of mainland Australia from the Sydney region to Adelaide and Kangaroo island.
Adult specimens reach 1.2 kg in body weight. The species is omnivorous and nocturnal and appears to breed all year round, producing as many as 4 litters of 2 or 3 young. Gestation length is probably less than 15 days, producing 350 mg newborn young that attach to a teat and remain in the pouch for a little under two months.
Methods
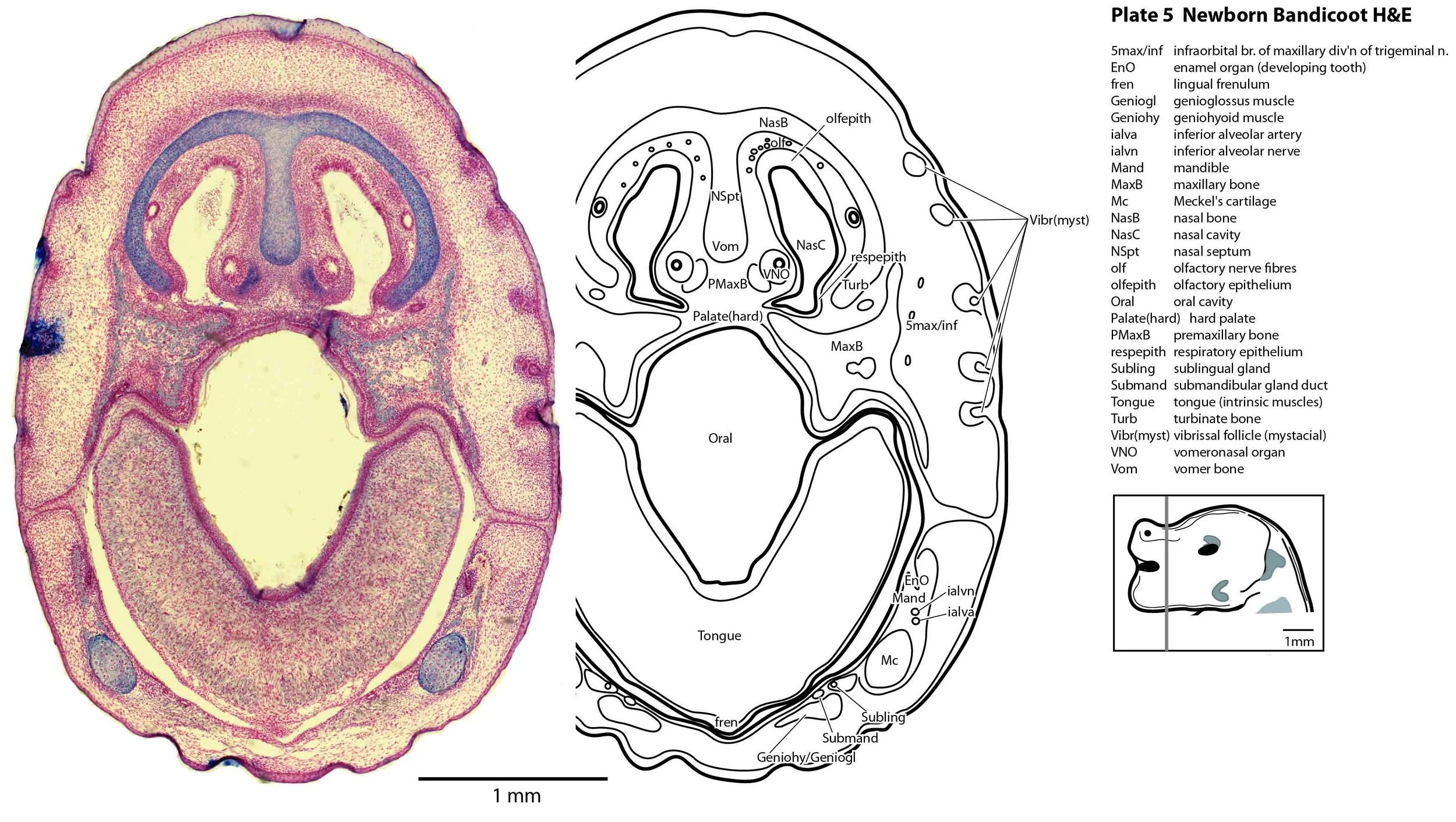
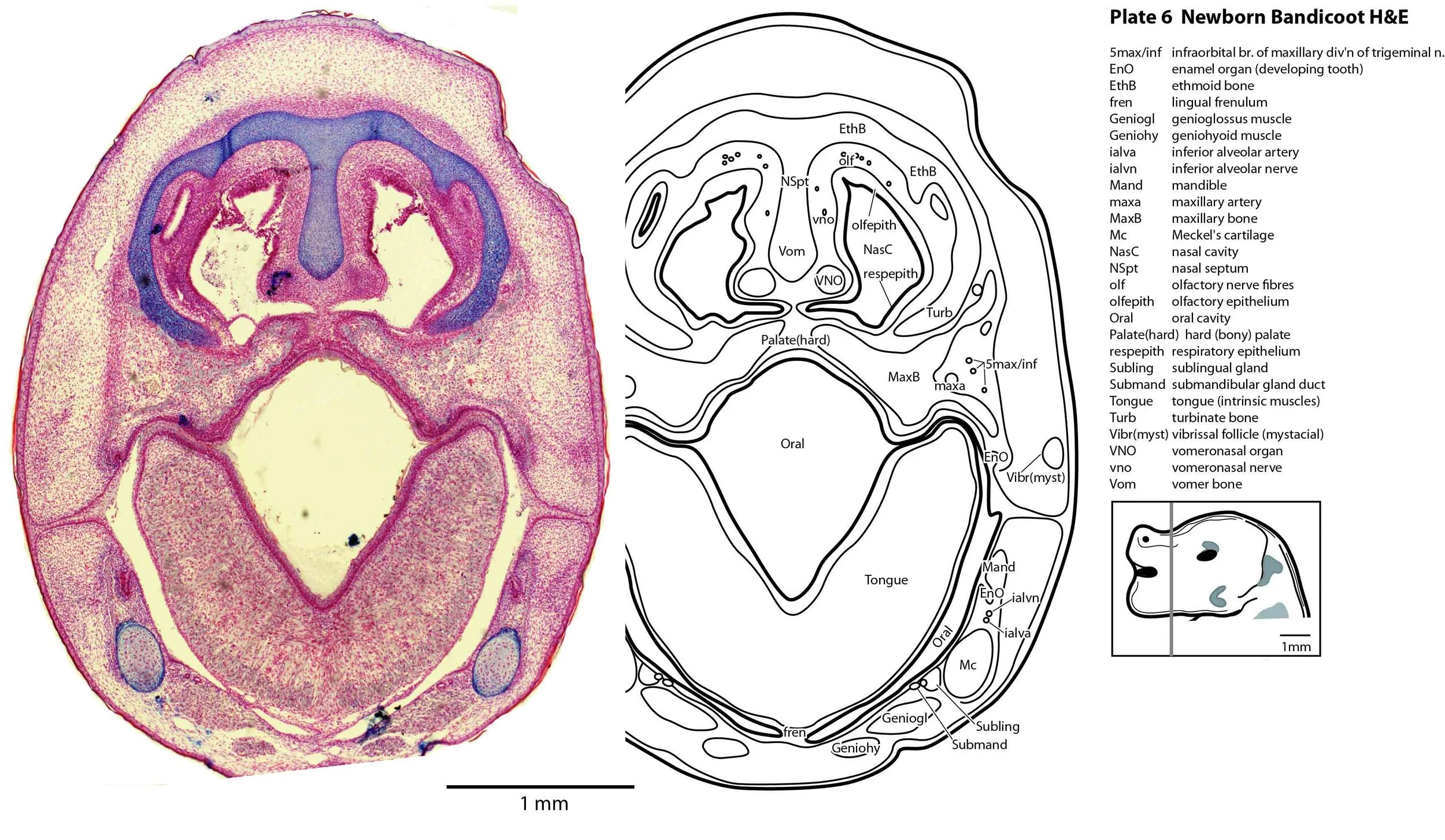
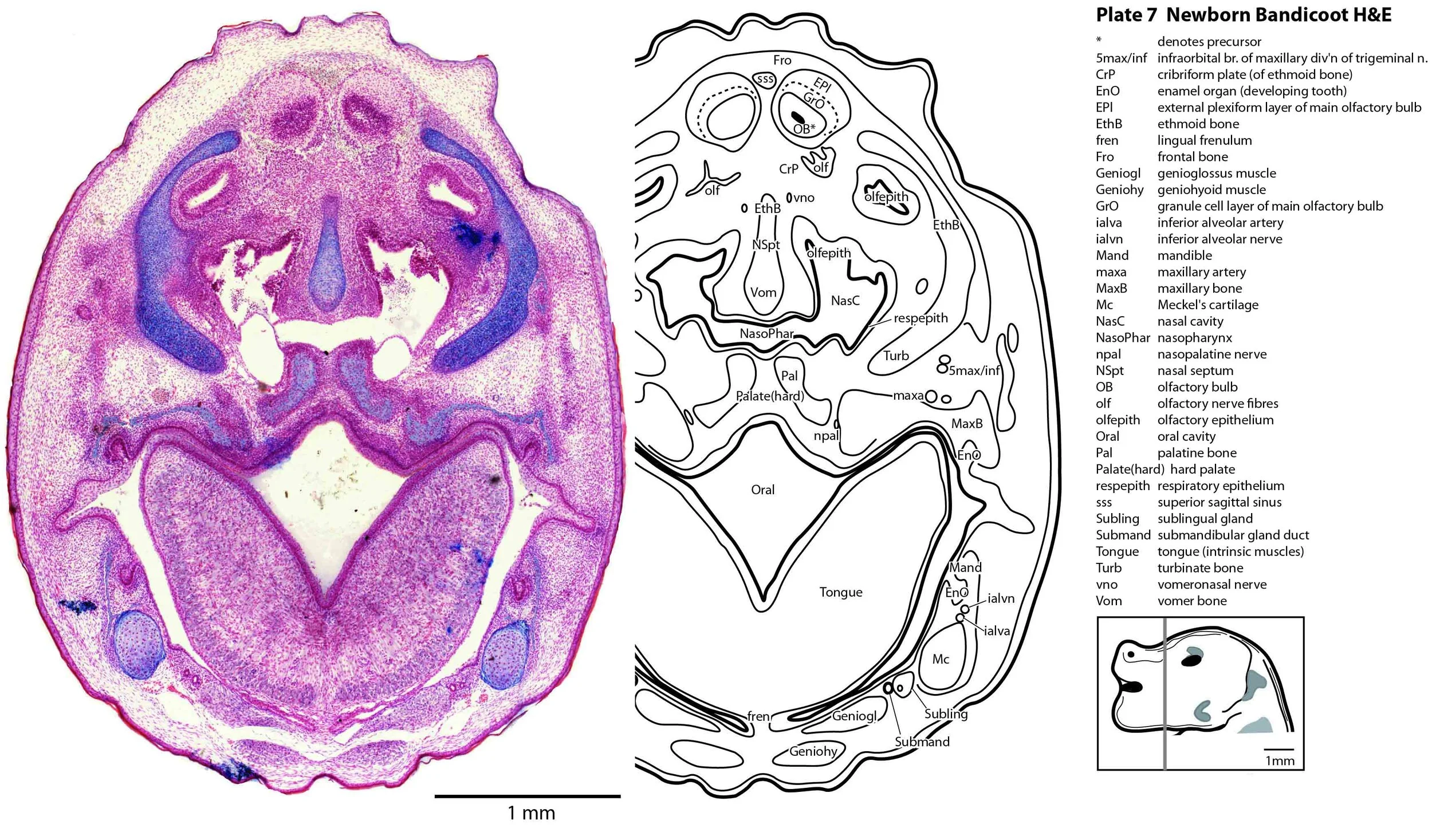
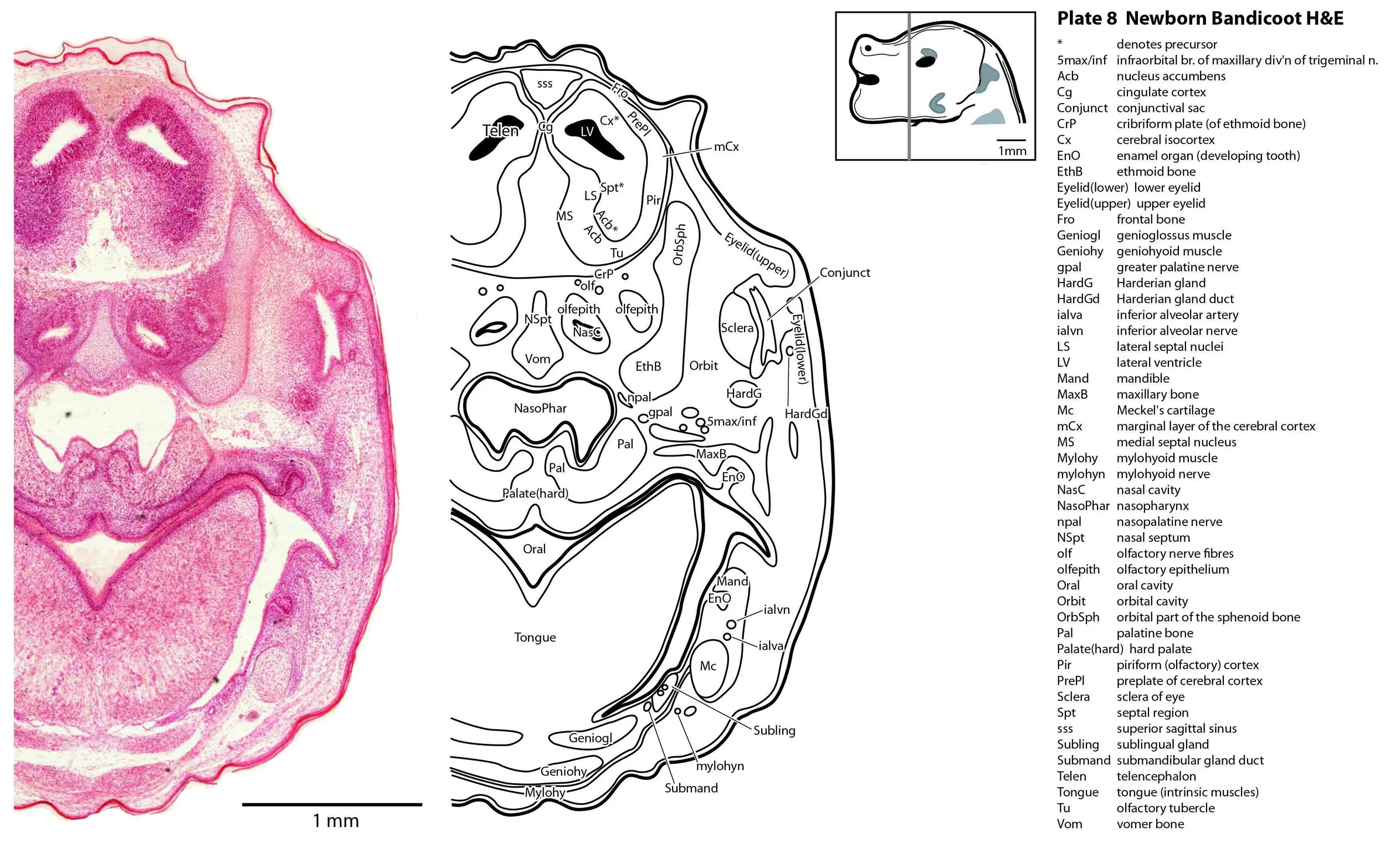
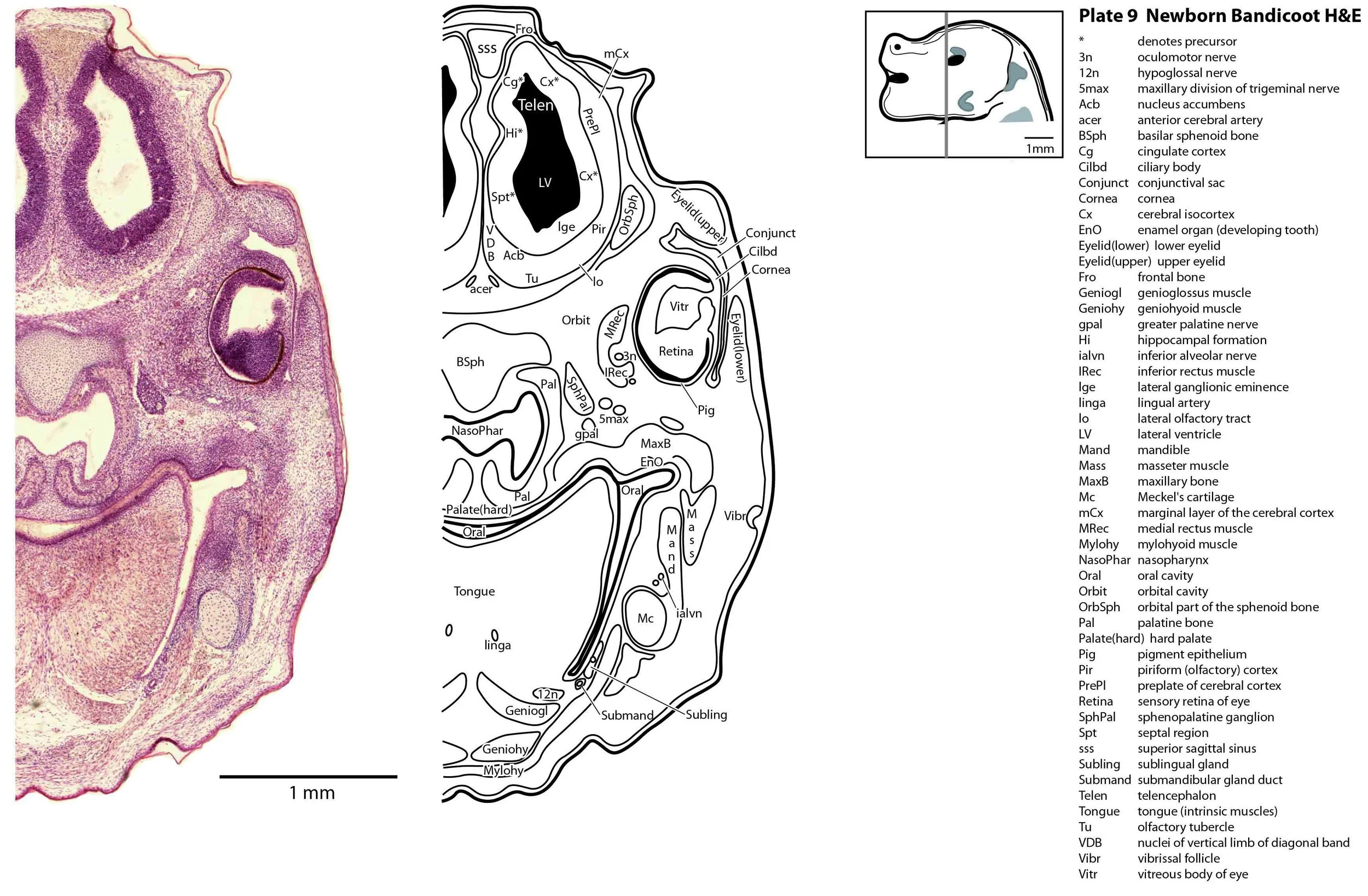
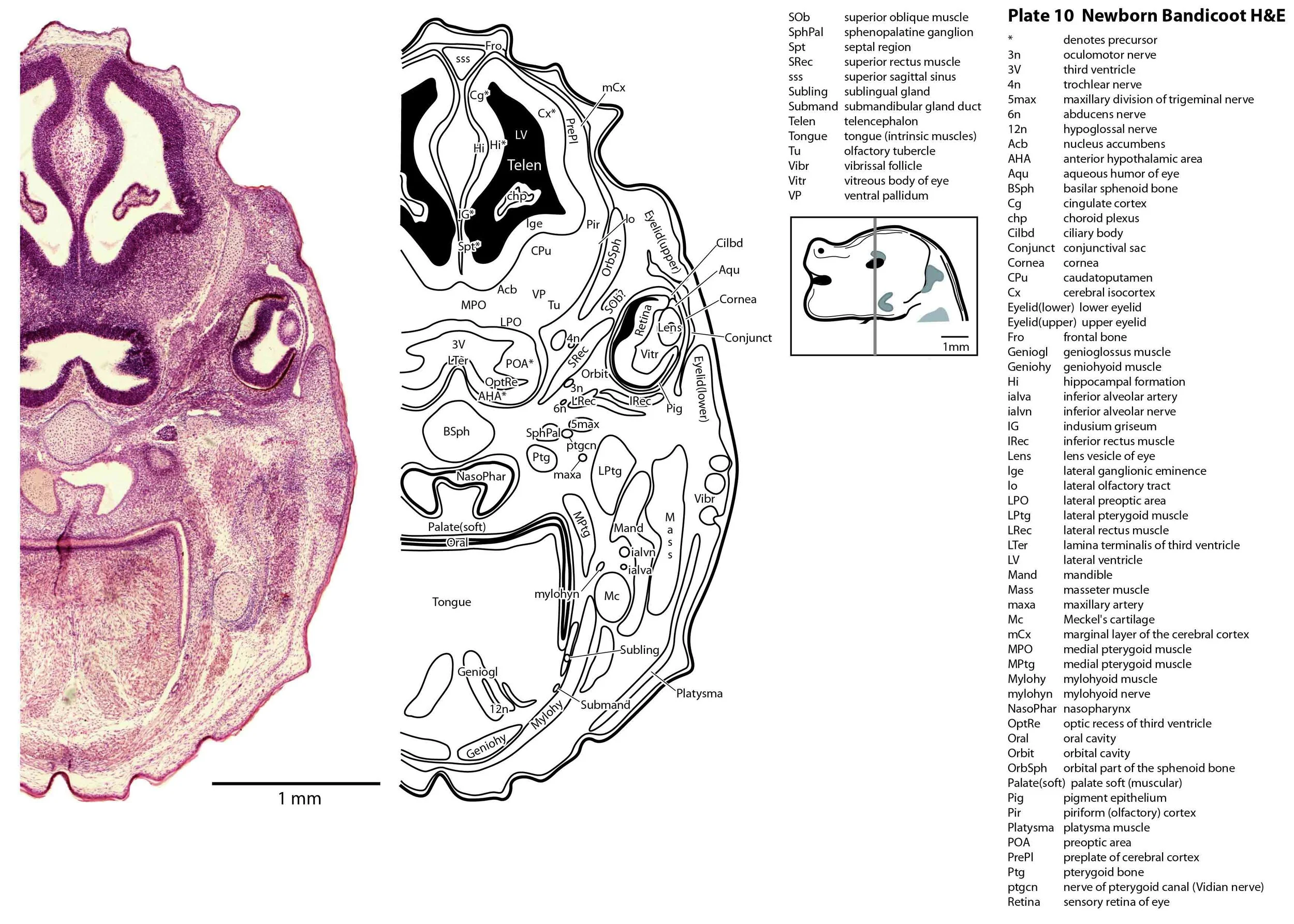
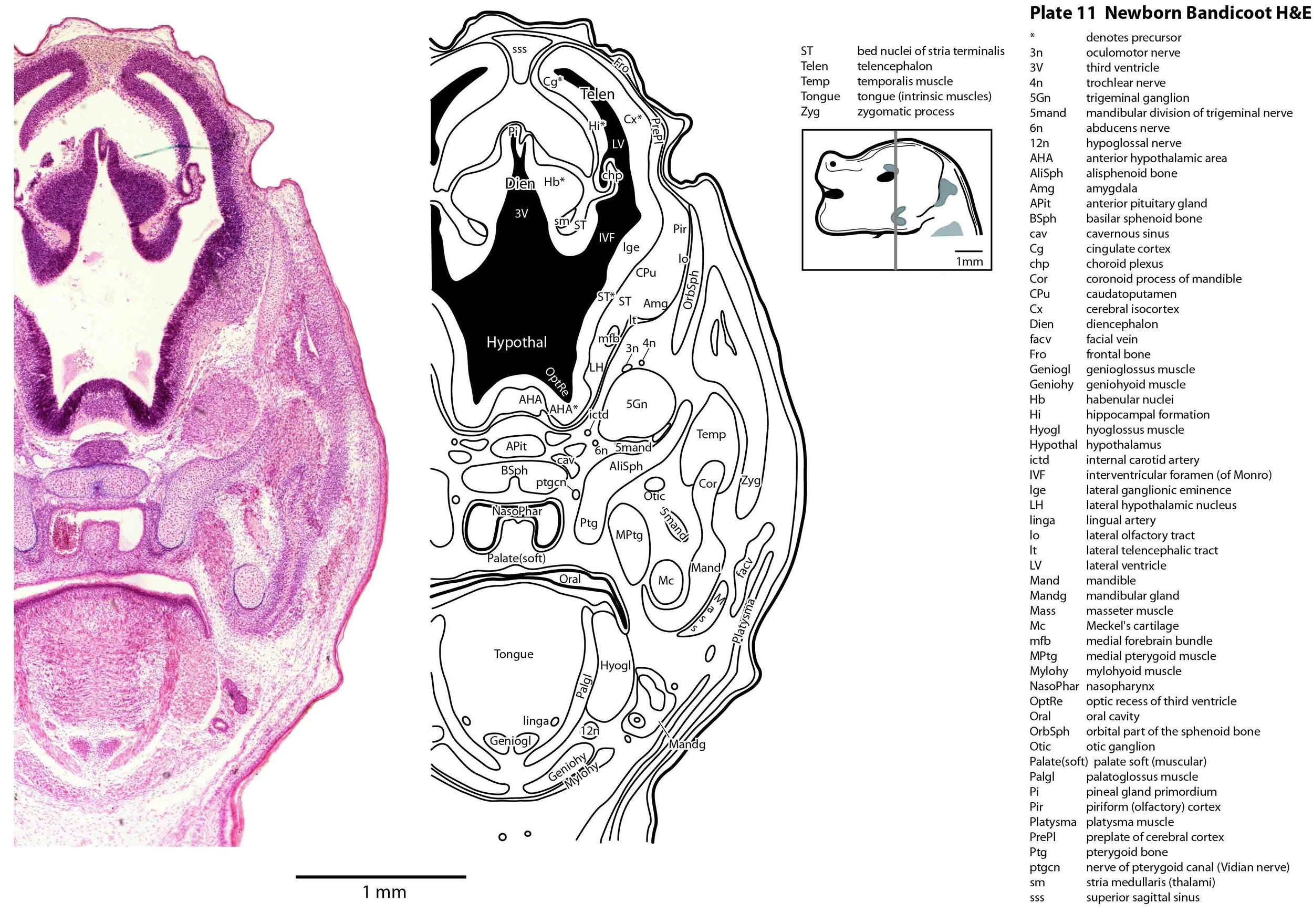
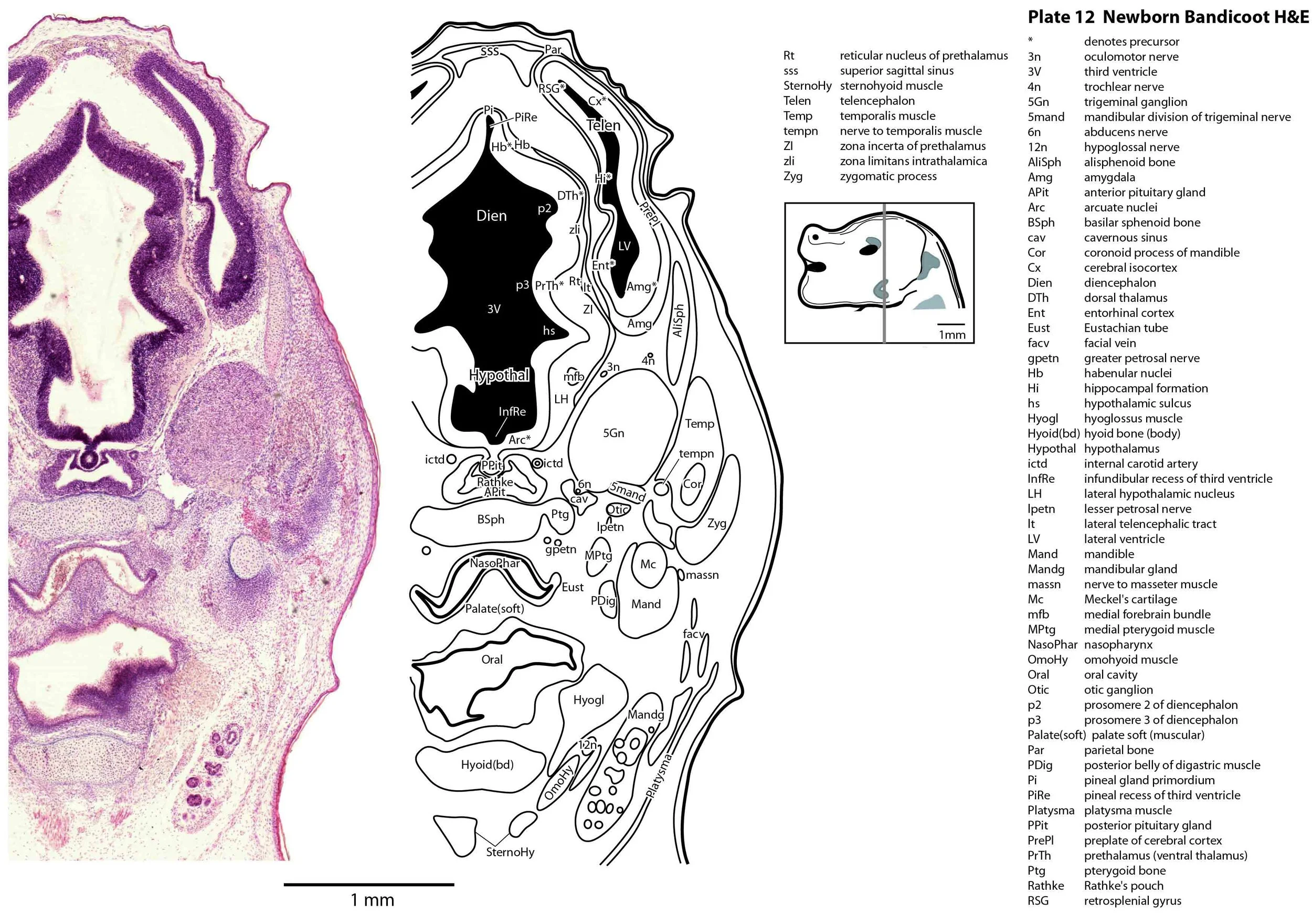
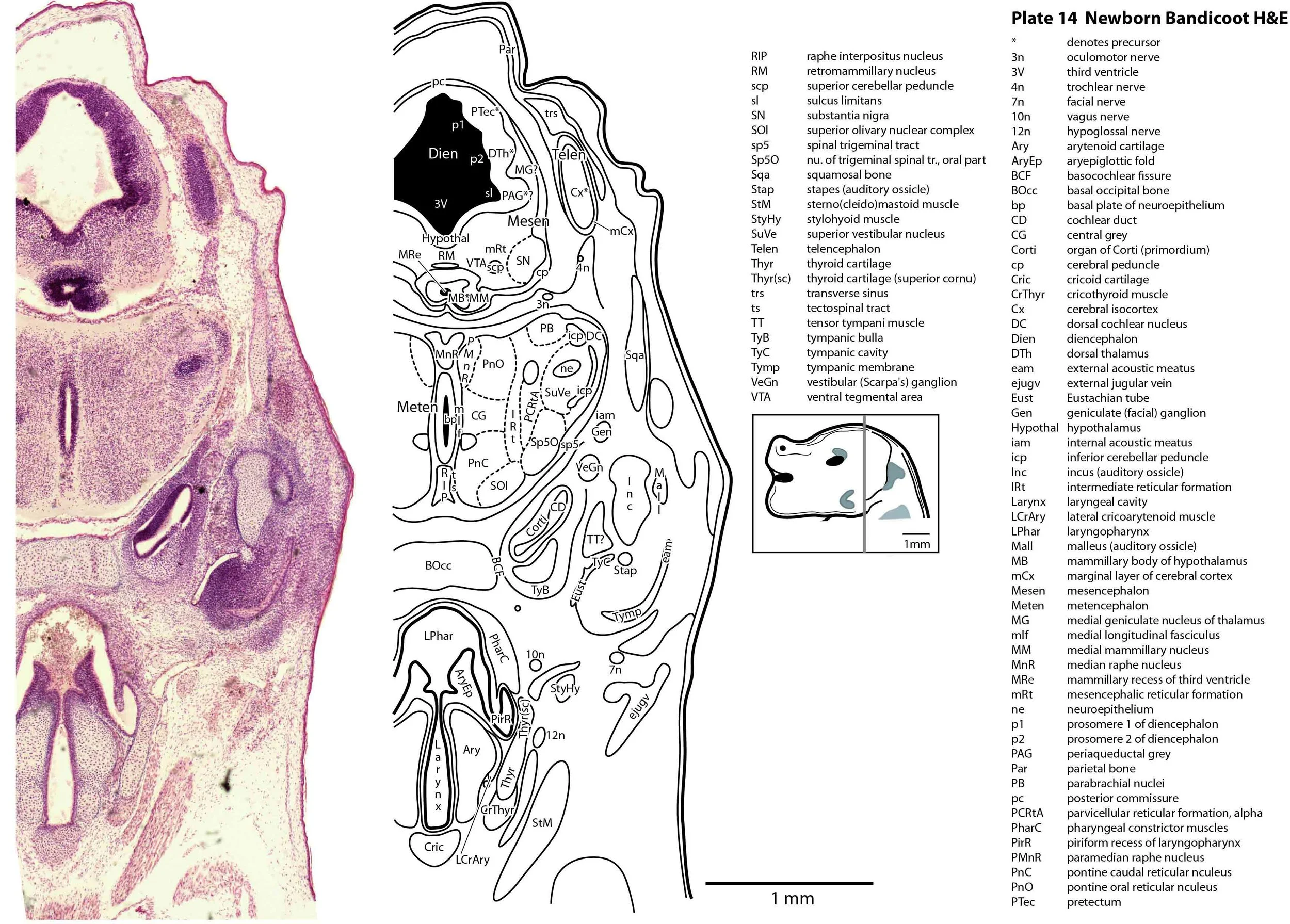
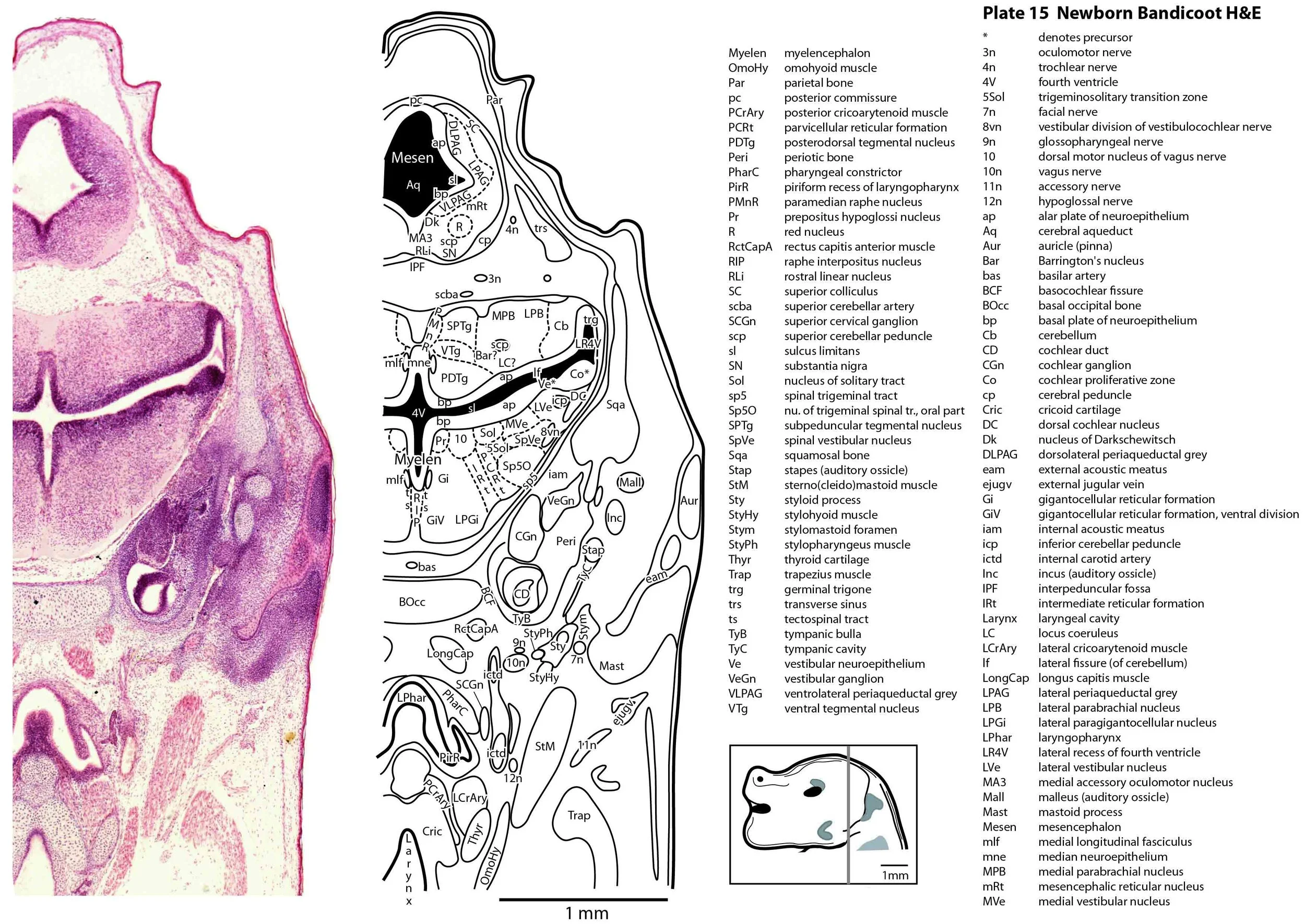
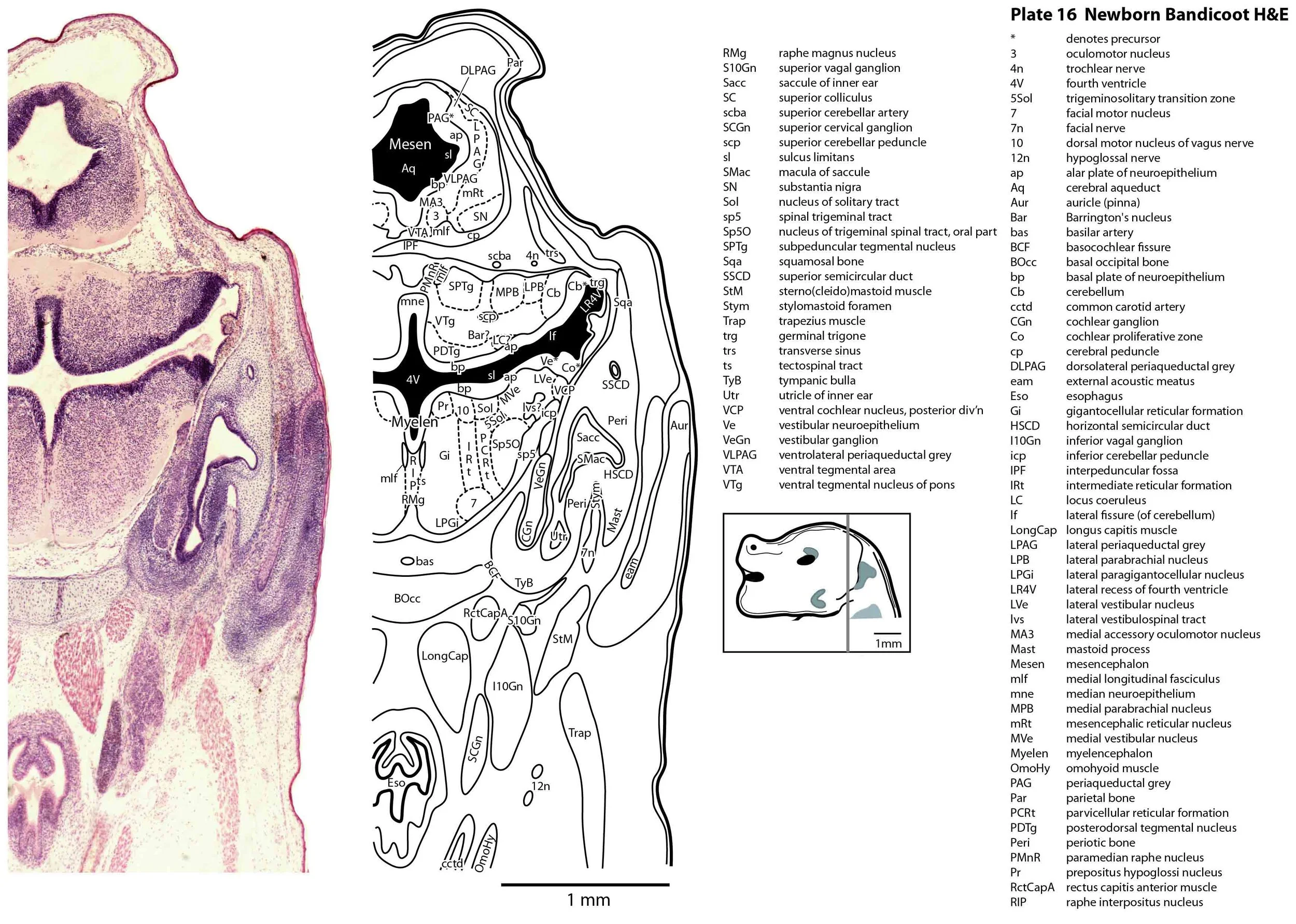
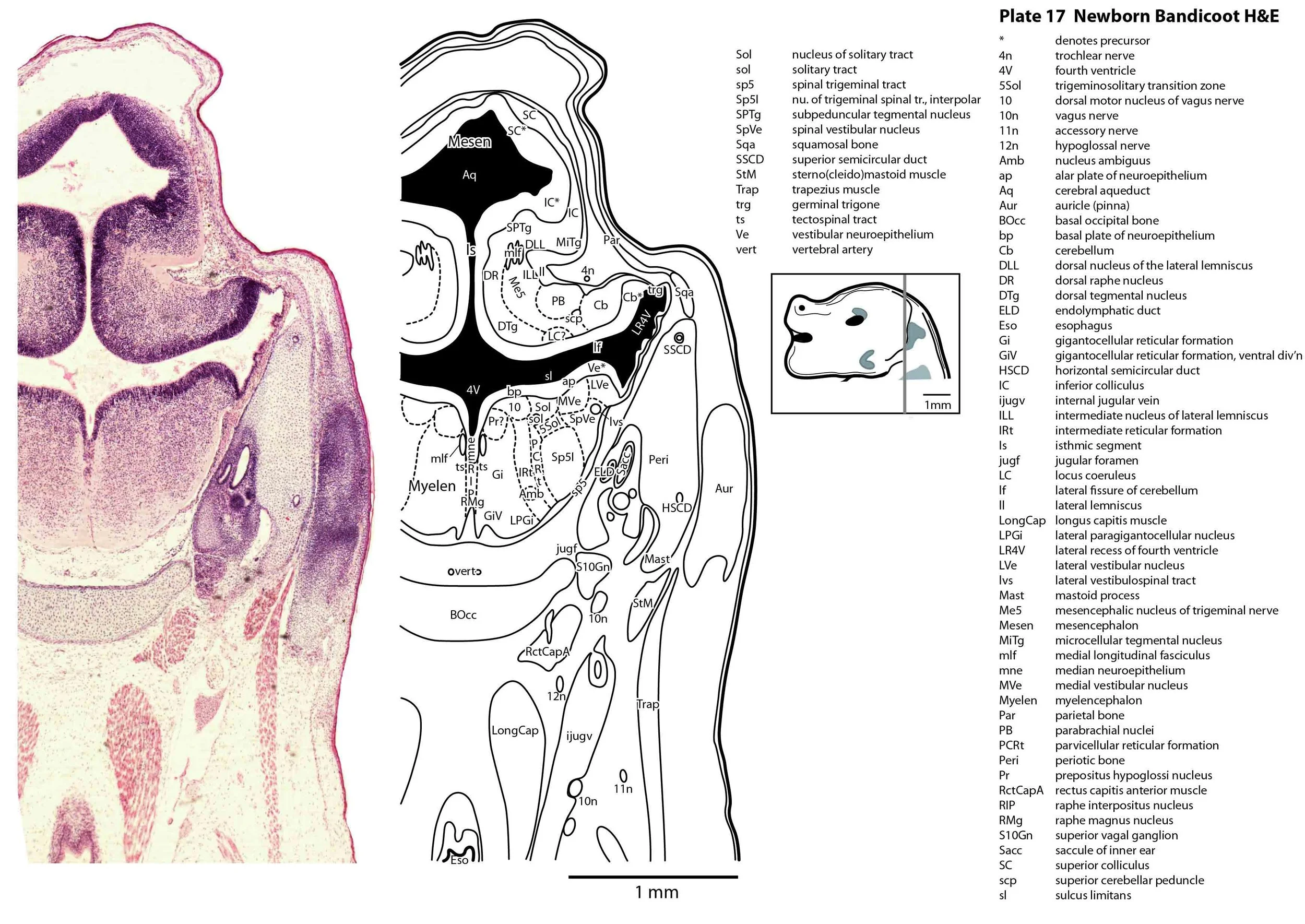
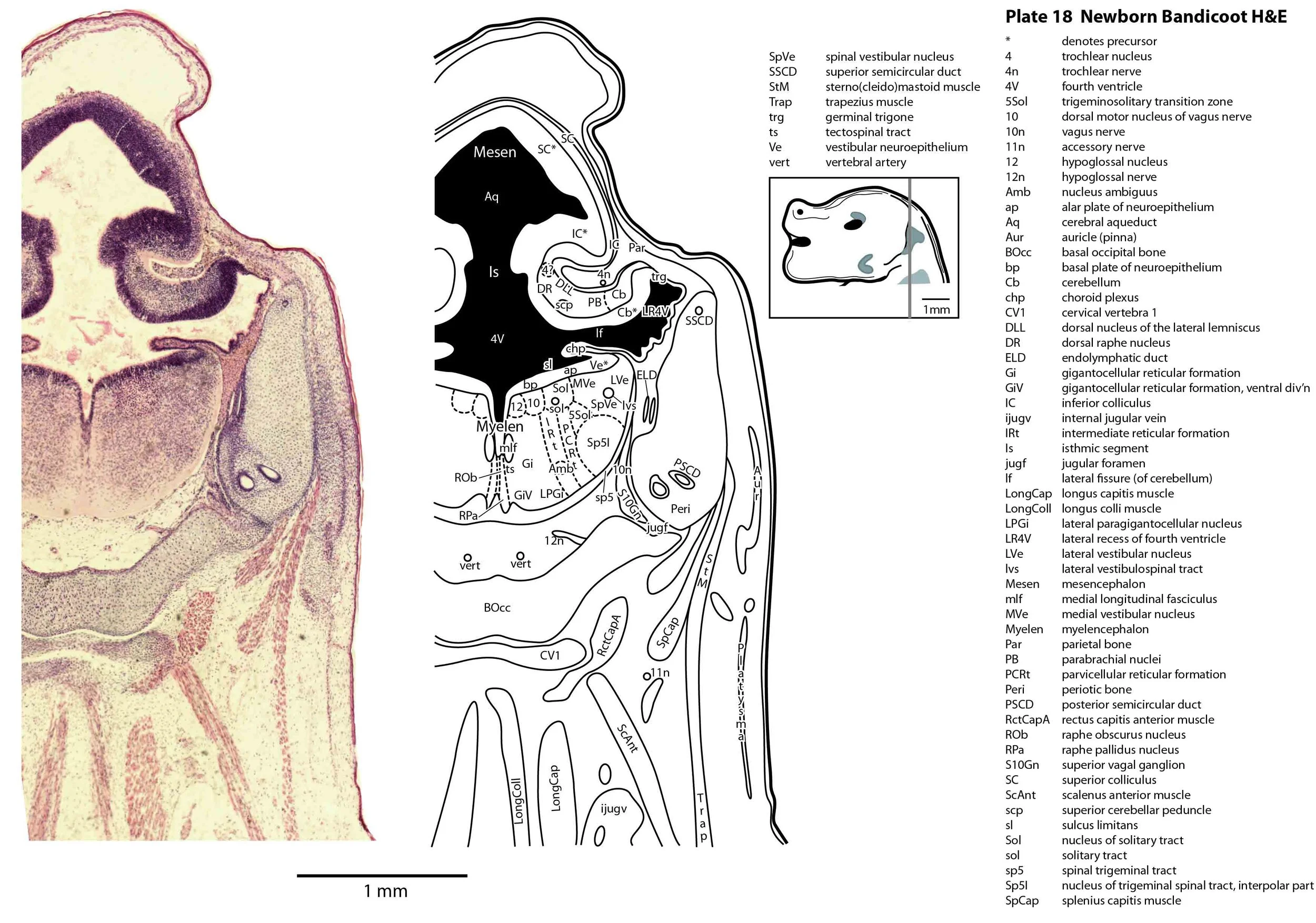
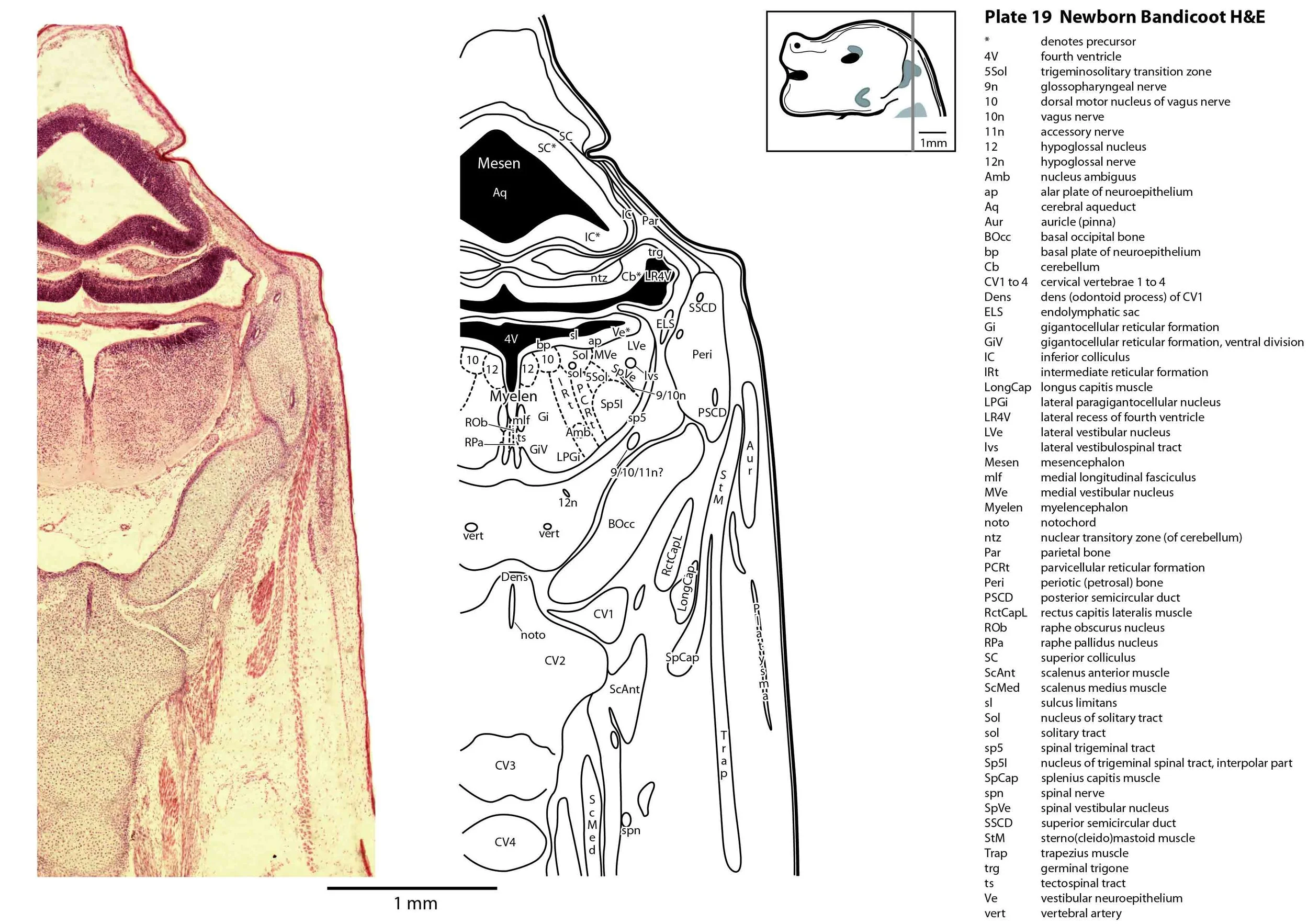
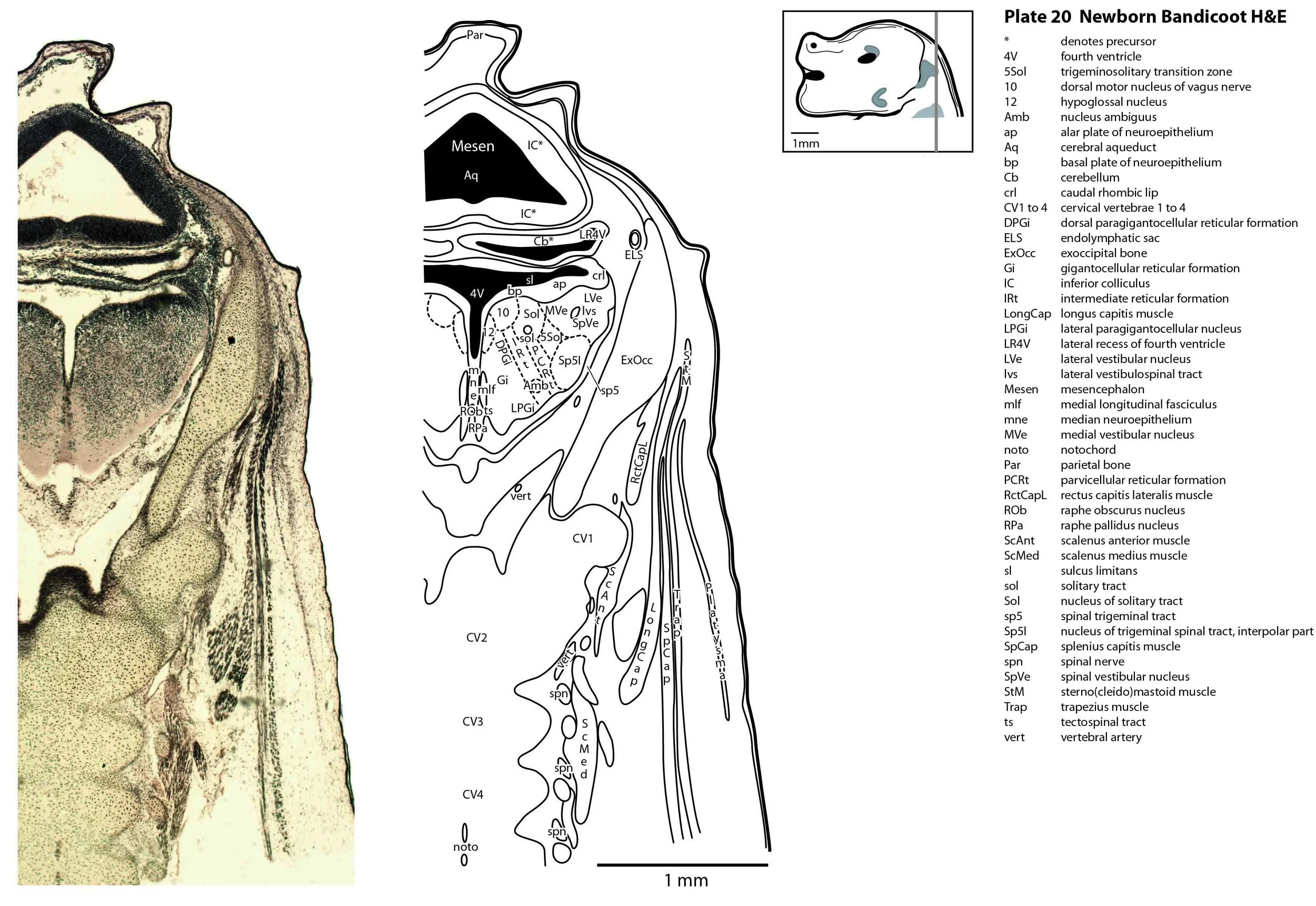
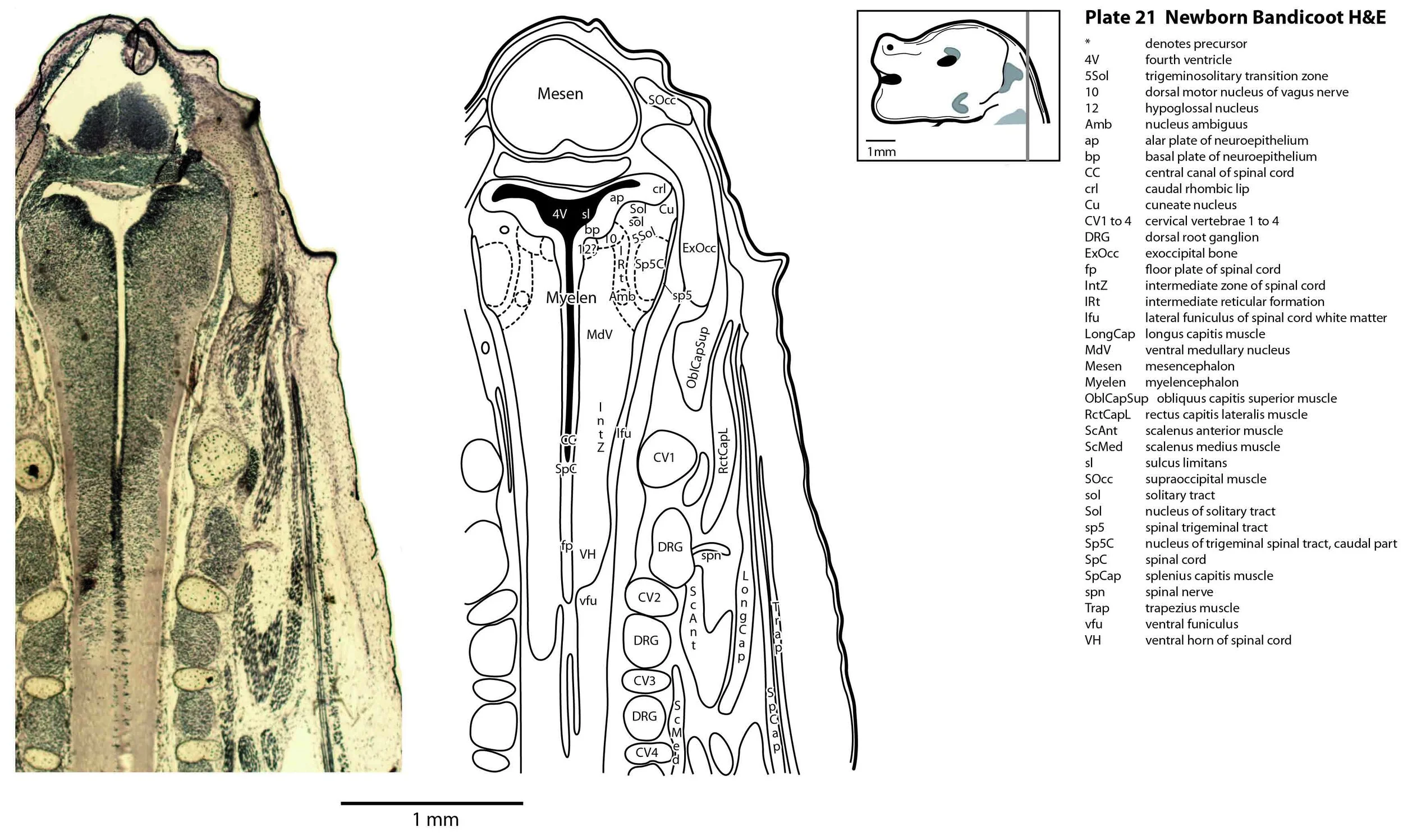
The specimen illustrated here is MS221 (Perameles obesula, newborn, GL of 15.5 mm, HL of 6.5 mm, 16 slides of sections) of the Hill collection stored at the Museum für Naturkunde in Berlin. The specimen had been collected in the late 19th century, embedded in paraffin wax and sectioned at 12 µm thickness, before being stained with hematoxylin and eosin as the predominant stain, with some sections apparently stained for alcian blue (hence the blue cartilage) or hematoxylin alone.
The sections at approximately 120 µm intervals were photographed with the aid of a Zeiss Axioplan2 fitted with an AxioCam MRc5 camera. All images were calibrated by photographing a scale bar at the same magnification. Images were placed in Adobe Illustrator 2021 and delineated. Developmental regions (i.e. neuroepithelium) destined to give rise to adult structures have been denoted by the adult structure’s name with an asterisk (e.g. Cx* denotes the developmental field of the cerebral isocortex).
Notes on the specimen
General observations
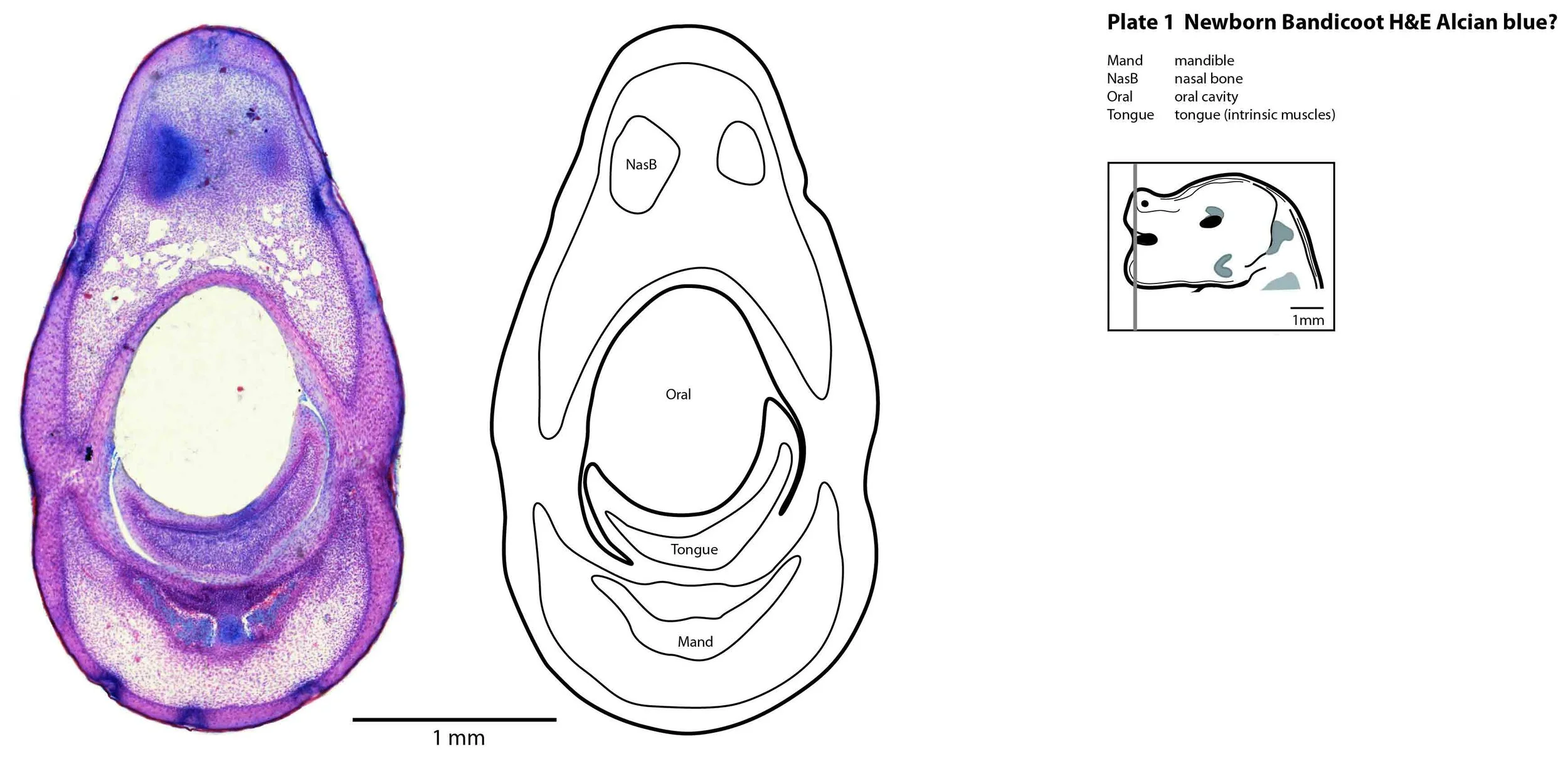
The oral shield (Schneider and Gurovich, 2017), which is an epidermal thickening anterior to the nasal, premaxilla, maxilla and dentary, is less prominent in this species (plates 1, 2) than in the newborn dasyurid. In general, the central nervous system, cranial skeleton and craniofacial musculature in the newborn of this species are intermediate in differentiation between those features in a newborn dasyurid (e.g. quoll) and a newborn macropod (e.g. tammar wallaby).
Central nervous system
The central nervous system is immature in this specimen compared to a newborn placental mammal such as the laboratory rat. The telencephalon has paired telencephalic vesicles (e.g. plates 10, 11) but the proliferative compartment known as the ventricular germinal zone (Cx*) is thicker than the preplate (PrePl) that contains the post-mitotic neurons. This is particularly the case in more caudal parts of the telencephalic vesicle (e.g. plates 13, 14) where the preplate is negligible. This suggests a distinct neurogenetic gradient in the developing bandicoot isocortex, such that rostral or anterior parts (frontal, motor, rostral somatosensory cortex) are in advance of caudal or posterior parts (caudal somatosensory, visual cortex).
Some olfactory nerve fibres are visible, but very few neurons are present in the olfactory bulbs, with only sparsely populated granule cell (GrO) and external plexiform (EPl) layers visible (plate 7). Infolding of the hippocampal primordium has begun (plate 10) but vey few neurons have been generated at this stage and no dentate gyrus primordium is visible.
The diencephalon (plates 12 to 14) can be divided into prethalamus (previously ventral thalamus), (dorsal) thalamus, and pretectum, derived from prosomeres 3, 2 and 1, respectively. Some white matter has begun to appear on the exterior of the forebrain. These include the stria medullaris thalami (sm; plate 11), lateral telencephalic tract (lt; plates 11 to 13) and cerebral peduncle (cp; plates 13 to 16). Both the lt and cp are substantially smaller than in a newborn tammar, but all white matter tracts in the newborn bandicoot are much larger than in a newborn quoll, highlighting the intermediate level of CNS maturation in this species.
The brainstem is more advanced in this species at birth than the newborn dasyurid, but not as differentiated as the newborn tammar wallaby. The trigeminal sensory nuclear complex (Pr5, Sp5O, Sp5I, Sp5C; plates 13 to 22) is easily distinguished and the medial magnocellular reticular formation (Gi, GiV, LPGi; plates 15 to 20) is broader that the parvicellular reticular formation (PCRt; plats 14 to 20). This would be consistent with a major role for trigeminal somatosensation in analysing the environment and feeding information to the brainstem reticulospinal pathways for co-ordination of forelimb movement.
The cerebellum is of a similar level of differentiation to an E14 rat (plates 15 to 20), with very few postmitotic cells in a nuclear transitory zone (ntz – plate 19) and only rudimentary inferior and superior cerebellar peduncles (plates 14 to 16, and 14 to 18, respectively). Precerebellar nuclei are also rudimentary, with neither the inferior olivary nor pontine nuclei present.
The spinal cord is most differentiated in the cervical region (e.g. plates 20 to 22), but even here there is very limited differentiation of the dorsal (sensory) horn.
Peripheral nervous system
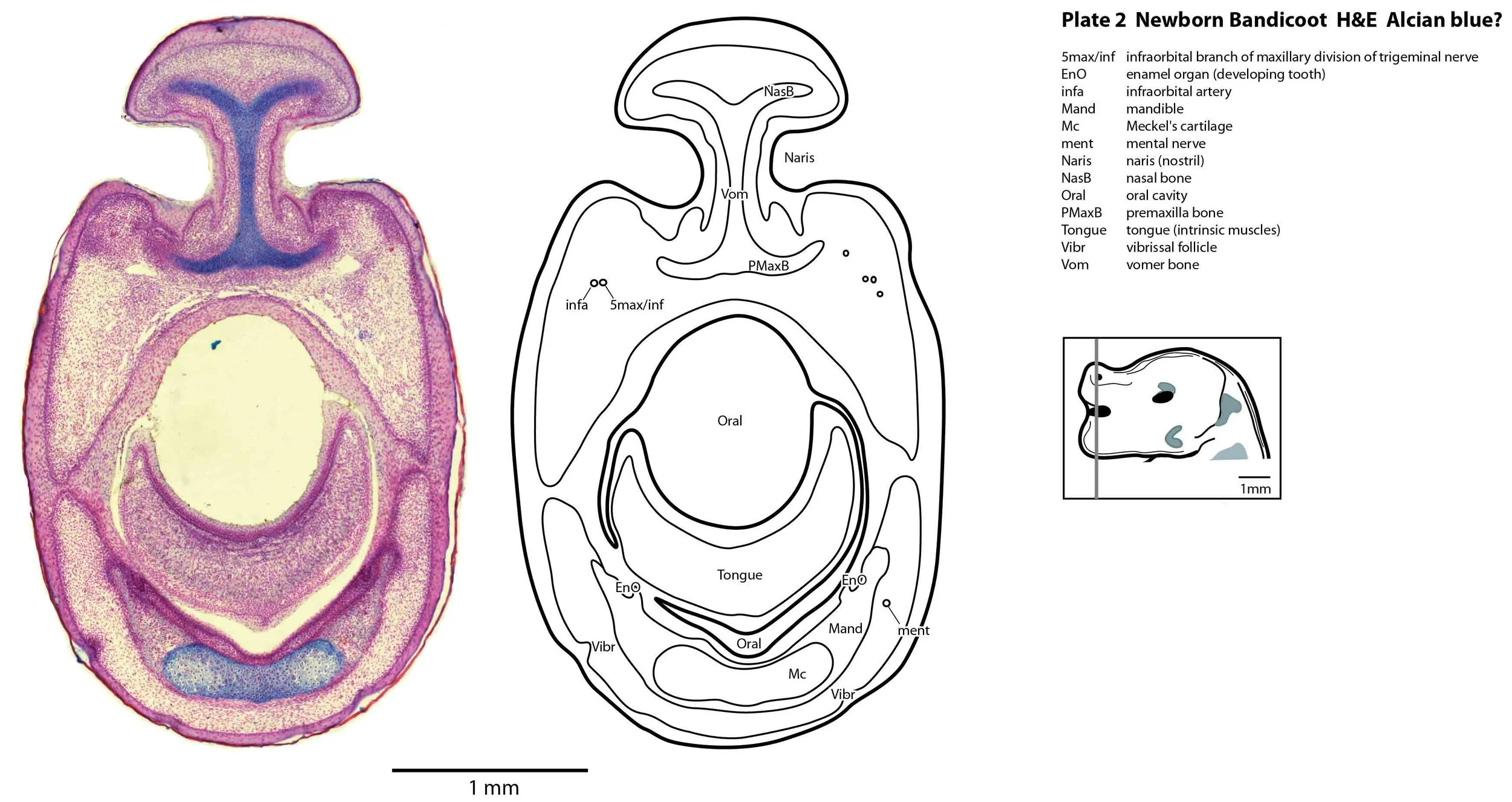
Most cranial nerves are visible (CN1 – olfactory in plates 5 to 8, CN3 – oculomotor in plates 9 to 15, CN4 – trochlear in plates 10 to 18, CN5 – trigeminal in plates 9 to 13, CN7 – facial in plates 14 to 16, CN8 – vestibulocochlear in plate 15, CN10 – vagal in plates 13 to 19, CN11 – spinal accessory in plates 15 to 19, CN12 – hypoglossal in plates 9 to 19). As with all marsupials, the most differentiated cranial nerve is the trigeminal, with all three divisions visible (ophthalmic, maxillary and mandibular) including some of the major branches of the maxillary and mandibular division (e.g. 5max/inf in plates 2 to 8; ialvn in plates 3 to 10).
Sense organs
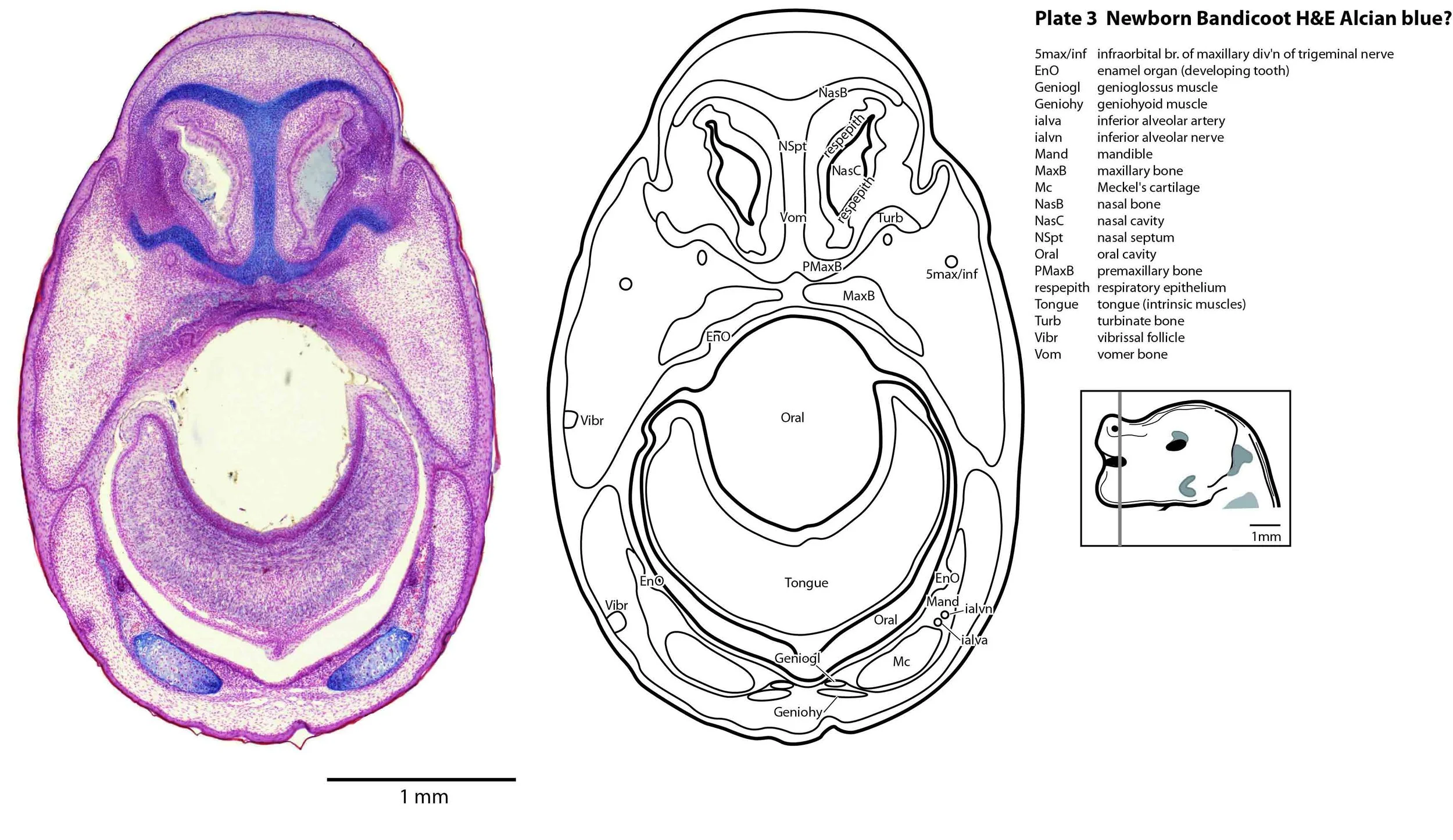
In the nasal cavity, the thicker olfactory epithelium can be distinguished from the thinner respiratory epithelium (plates 5 to 8), but the rest of the olfactory system is still embryonic (see main olfactory bulb discussed above and in plate 7).
The eyes are only rudimentary (plates 9, 10). A conjunctival sac, cornea and lens can be identified, but the optic cup is small. A proliferative compartment in the sensory retina has appeared (plates 9, 10), but very few neurons have been generated. No optic nerve is visible.
The external acoustic meatus is uncanalised (plates 14 to 16) and the Eustachian (pharyngotympanic) tube has just reached the region of the future middle ear (plates 12 to 14). The otic vesicle has an endolymphatic duct and sac (plates 17 to 20) visible as an elongated dorsally directed diverticulum. Other subregions (utricle, saccule, cochlear duct and semicircular ducts) are also distinguishable (plates 14 to 19), but sensory maculae are poorly differentiated. As regards middle ear bones, a putative stapes is visible (plates 14, 15), along with cartilaginous models of the incus (plates 14, 15) and malleus (plates 14, 15).
Early cutaneous sense organs in the form of vibrissae (mystacial – plates 5, 6; and other types – plates 2 to 4, 9, 10) are seen, but the lingual epithelium does not show any taste buds.
Musculoskeletal system
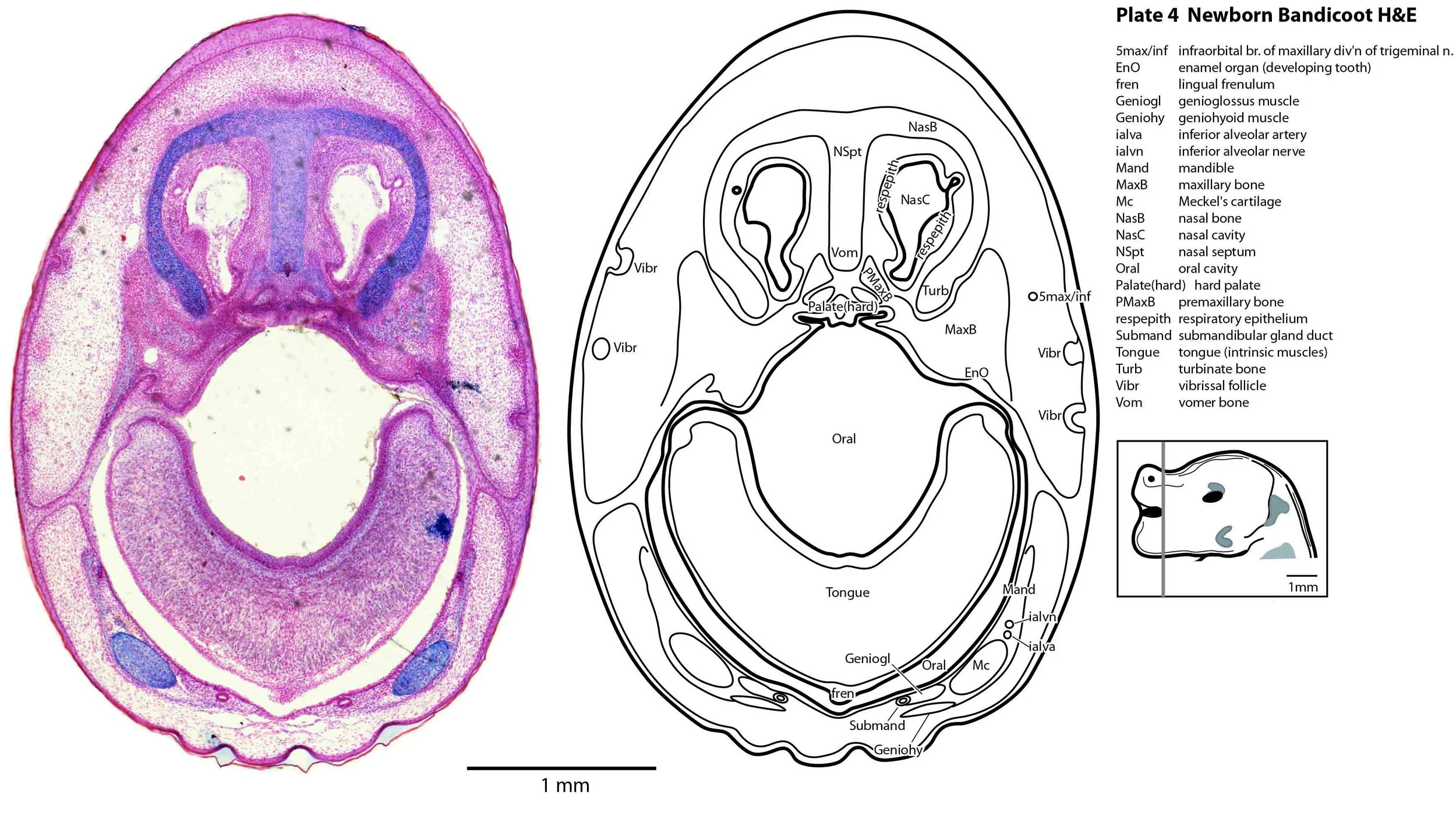
Cartilaginous models for the premaxilla, nasal, turbinate, ethmoid, maxillary, basal sphenoid, basal occipital, squamosal, periotic, ex- and supraoccipital bones are present. The mandible (dentary) is developing around the Meckel’s cartilage (Plates 2 to 13).
Muscles have been identified by reference to Diogo et al. (2016). Of the facial muscles, only the platysma (plates 10 to 13), digastric (plate 12) and stylohyoid (plates 13 to 15) are visible. There is no musculature associated with the developing mystacial vibrissae. Pterygoid (plates 10 to 13), temporalis (plates 11 to 13) and masseteric (plates 9 to 11) masticatory muscles are also present. Intrinsic and extrinsic tongue musculature is present (plates 1 to 11), along with stylopharyngeus (plate 15) and salpingopharyngeus (plate 13) muscles inserting into the pharyngeal constrictor tube (plates 14, 15). Three intrinsic laryngeal muscles (lateral cricoarytenoid – plates 14, 15; posterior cricoarytenoid – plate 15; and cricothyroid – plates 13, 14) are visible.
Acknowledgements
I would like to thank Dr Peter Giere of the MfN, Berlin Germany, for access to the collection and for all his kind help during the work.
References
Diogo R, Bello-Hellegouarch G, Kohlsdorf T, Esteve-Altava B, Molnar JL (2016) Comparative myology and evolution of marsupials and other vertebrates with notes on complexity, bauplan, and “scala naturae”. Anat Rec 299, 1224-1255.
Schneider NY, Gurovich Y (2017) Morphology and evolution of the oral shield in marsupial neonates including the newborn monito del monte (Dromiciops gliroides, Marsupialia Microbiotheria) pouch young. J Anat 231, 59-83.