Atlas of the Forebrain of a Newly Hatched (16.75 mm GL) Platypus (M44)
Introduction
This series of images illustrates the limited extent of forebrain development (especially the cerebral cortex and the thalamus) at hatching.
A platypus of 16.75 mm GL (greatest length) would be no more than 1 day after hatching (Ashwell, 2013), where hatching occurs at about 14 to 16 mm GL.
Details on platypus biology can be found at the Platypus page of this website.
Methods
The specimen illustrated here is M44 (a newly hatched platypus) of the Hill collection, stored at the Museum für Naturkunde in Berlin. The most likely site of collection is eastern New South Wales, near Sydney. Note that the specimen card states 16.45 mm GL (newly hatched), but the slides state 16.75 mm GL.
The specimen had been collected in the late 19th century by JP Hill, embedded in paraffin wax and sectioned transversely (frontally) at 10 µm thickness. The stain was not identified on the specimen card, but it appears to be a variant of hematoxylin stain.
Sections at approximately 70 µm intervals were photographed with the aid of a Zeiss Axioplan2 fitted with an AxioCamMRc5 camera. All sections were photographed with a 2.5x objective and images were calibrated by photographing a scale bar at the same magnification. Images were placed in Adobe Illustrator 2021 and delineated.
General observations
The forebrain can be divided into developmental segments according to the schema of Puelles and co-workers (Puelles et al. 2004; Puelles et al. 2012a, b). These include the three prosomeres (prosomere 1 – pretectum, prosomere 2 – thalamus, prosomere 3 – prethalamus; see plates 12 to 19), the hypothalamus (plates 8 to 19), and the telencephalon (including cerebral cortex or pallium, and subpallial structures such as the basal ganglia and septal nuclei; see plates 1 to 19).
Cerebral cortex
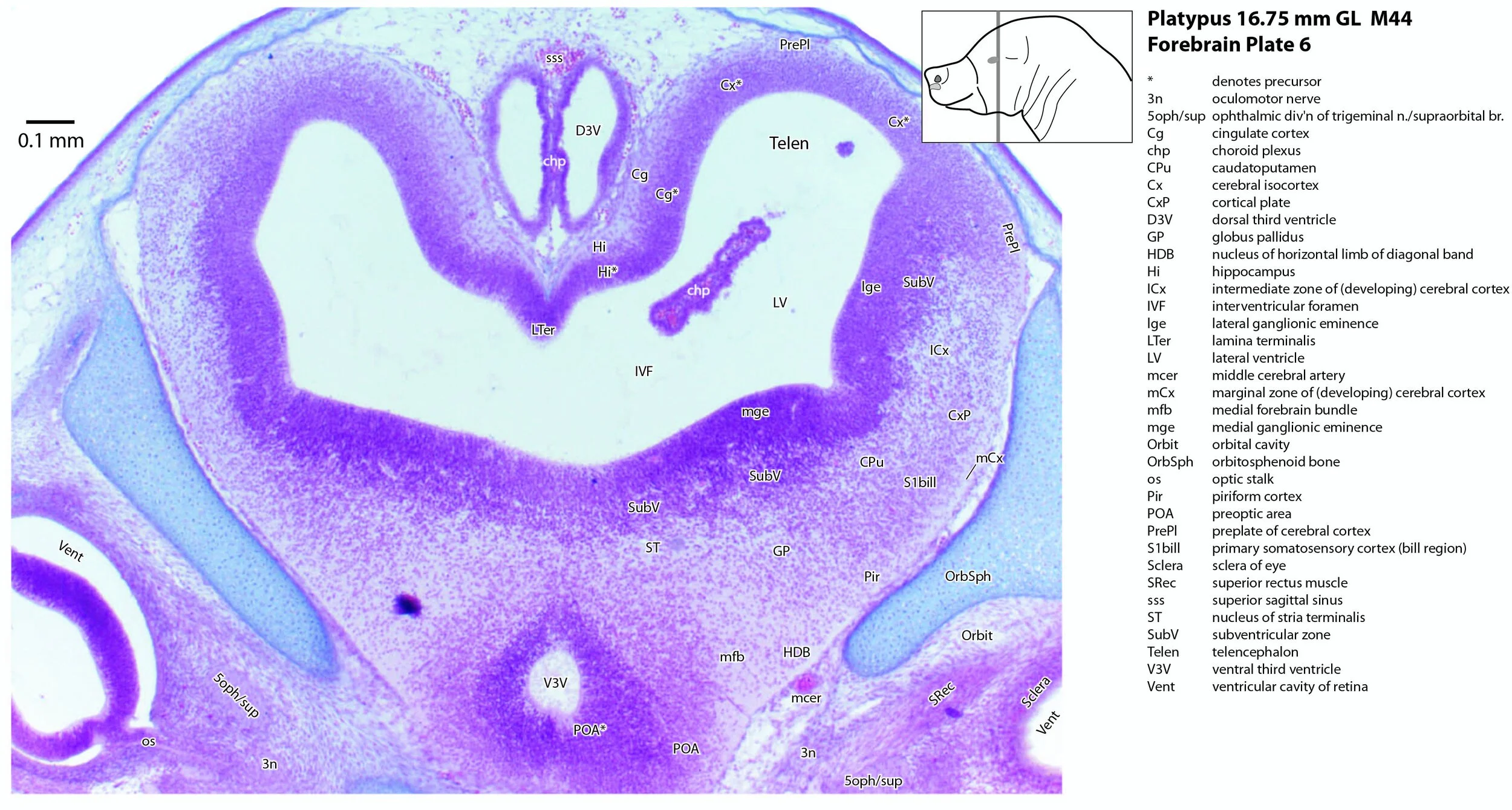
The cerebral cortex (Cx – plates 1 to 19) or pallium is relatively thin at this developmental stage, with little or no postmitotic neurons evident across the dorsomedial and dorsolateral curvature. Only the piriform (primary olfactory) cortex (Pir – plates 2 to 13) and the most ventrolateral part of the primary somatosensory isocortex, where the bill is represented (S1Bill – plates 6 to 12), have cortical plates (CxP – plates 4 to 8) of any significant thickness.
The hippocampus (Hi – plates 4 to 11) is very poorly differentiated at this stage. Most of the region’s tissue is still neuroepithelial (i.e. dividing cells) and very few neurons have left the cell cycle. The fornix (outflow tract of the hippocampus) was not seen.
Subpallial parts of the telencephalon
Putative parts of the amygdala, caudatoputamen and septum have been indicated, but these are necessarily notional because nuclear boundaries are indistinct.
(Dorsal) thalamus
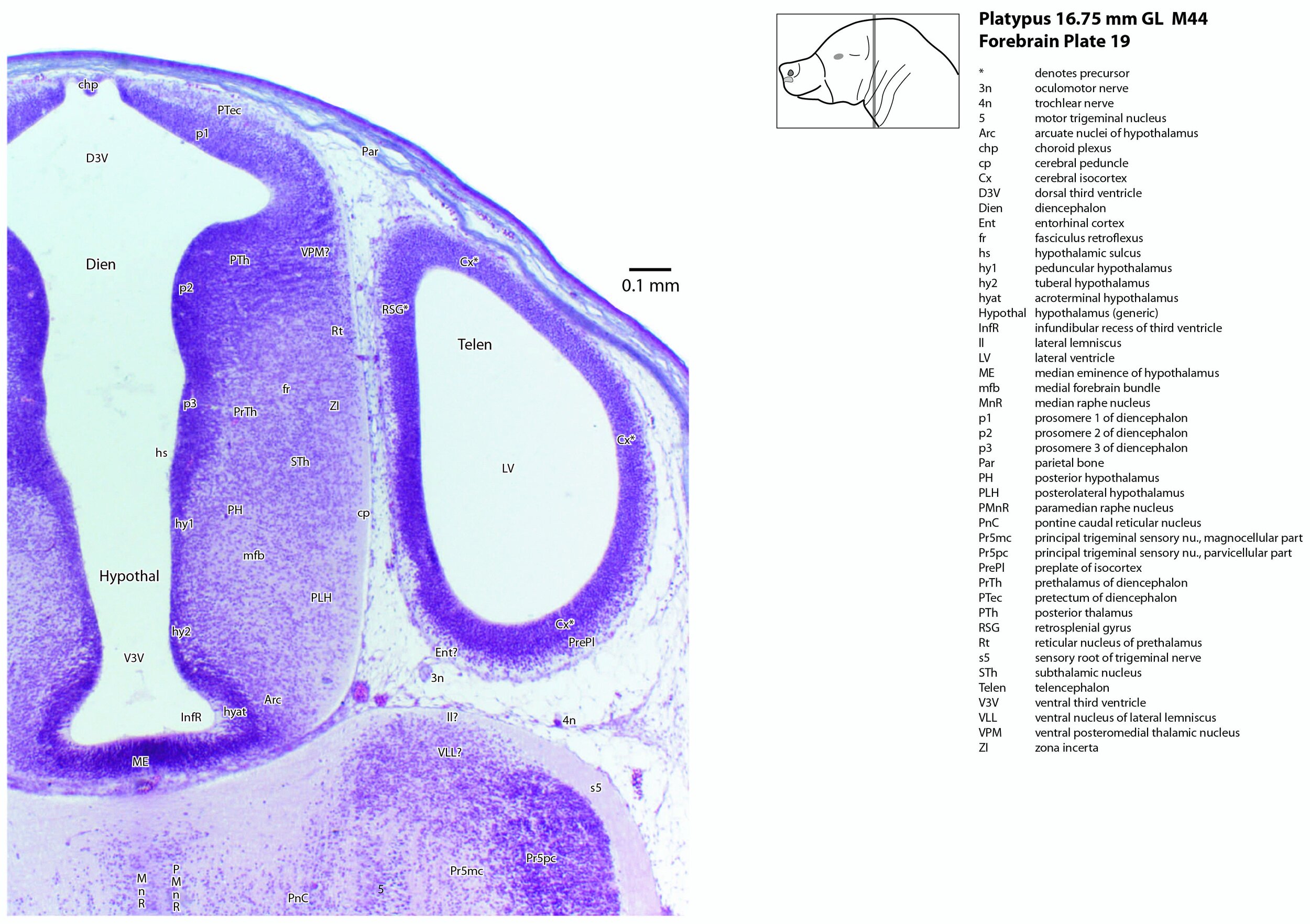
The thalamus (or more properly, the dorsal thalamus) is represented by anterior and posterior regions (ATh – plates 9 to 11; PTh – plates 12 to 19) and has a thick neuroepithelium with only few postmitotic neurons. A putative ventral posteromedial nucleus (for head somatosensory representation; VPM – plates 17 to 19) has been tentatively identified.
Olfactory apparatus
The rostral olfactory system has already been commented on in the atlas of the bill of this specimen. The olfactory bulb is visible (OB – plate 1), but lamination is indistinct. A thin lateral olfactory tract (lo – plates 2 to 5) can be followed towards the primary olfactory cortex (Pir; see above).
Hypothalamus
Developmental subdivisions of the hypothalamus (peduncular, tuberal and acroterminal hypothalamus; hy1, hy2, hyat, respectively) described by Puelles and co-workers (Puelles et al. 2012a) have been identified here. As in other mammals, neurons of the most lateral parts of the hypothalamus (e.g. LH – plates 9 to 16) are the first to leave the mitotic cycle. There is a shallow hypothalamic sulcus (hs – plates 9 to 19) separating the hypothalamus from the diencephalon. The medial forebrain bundle (mfb – plates 5 to 19) can be traced rostrocaudally throughout the hypothalamus.
Acknowledgements
I would like to thank Dr Peter Giere of the MfN, Berlin Germany, for access to the collection and for all his kind help during the work.
References
Ashwell KW (2013) Embryology and post-hatching development of the monotremes. In KWS Ashwell (Ed.), Neurobiology of Monotremes: Brain Evolution in Our Distant Mammalian Cousins (1st ed., pp. 31-46). CSIRO.
Puelles L, Martínez S, Martínez-de-la-Torre M, Rubenstein JLR (2004) Gene maps and related histogenetic domains in the forebrain and midbrain. In The Rat Nervous System 3rd edn (Ed G Paxinos) pp. 3–125. Elsevier, San Diego.
Puelles L, Martinez-de-la-Torre M, Bardet S and Rubenstein JLR (2012a) Hypothalamus. In The Mouse Nervous System(Eds C Watson, G Paxinos and L Puelles) pp. 221–312. Elsevier, San Diego.
Puelles L, Martinez-de-la-Torre M, Ferran J-L and Watson CRR (2012b) Diencephalon. In The Mouse Nervous System(Eds C Watson, G Paxinos and L Puelles) pp. 313–336. Elsevier, San Diego.