A Cytoarchitectonic Atlas of the Brain of the Florida Manatee (Trichechus manatus latirostris)
Image by Benjamin Brant from Pixabay
Materials and Methods
The brain depicted here is from the Welker Collection (Welker Trichechus manatus, 84-49) and is also displayed online at BrainMuseum.org. Please see that website for photographs of the intact brain, which have been used for the finder diagrams used in the plates. The Nissl-stained sections are held at the National Museum for Health and Medicine in Silver Springs, Maryland, USA. Selected frontal plane sections in a regular sequence (please see section numbers on plates) were scanned with the aid of an Epson 11000.
The images were cropped and adjusted for optimal contrast levels with Adobe Photoshop (2021), before 18 sections were placed into Adobe Illustrator (2021) files for mapping. Only one side of the brain (right side of the section) has been depicted to maximise the detail visible online. Regions the brain were outlined and labelled using terminology adapted from those used in the Paxinos and Watson system of brain atlases. The scale in all images is in millimetres and the section number is given in the top left-hand corner of the plate. A small finder image in the top right-hand corner of each atlas plate shows the position of the frontal section (grey line) within the brain. Finished atlas plate files were exported to jpg for publishing.
The Manatee Brain
The brain of the adult Florida manatee weighs approximately 360 g, with a body weight of 400 to 550 kg, giving the species one tenth the encephalization of the great apes. The cortical surface is smooth (lissencephalic) like the dugong (Pirlot and Kamiya, 1985), with a relatively shallow lateral fissure (lf), and very shallow rhinal fissure (rf) and cingulate sulcus (cgs) (e.g. see Plate 7). Gyrification index is only 1.06 (Reep and O’Shea, 1990). The lateral ventricle (LV) is very large (e.g. see Plates 3 to 11), with a small extension into the temporal lobe superior to the amygdala. The cerebellar hemispheres are large, suggesting an expanded pontocerebellum for the trigeminal somatosensation required for oripulation.
The Manatee Cerebral Cortex
The delineation and nomenclature of the manatee cerebral cortex have been adapted with modifications from Sarko and Reep (2007). Nomenclature for regions which have obvious topographical relationships and cytoarchitectural similarities to cortical regions identified in rodent, carnivore and primate cerebral cortex (e.g. orbital, cingulate, infralimbic, entorhinal, ectorhinal, perirhinal, retrosplenial) has been adopted to reflect those similarities. The isocortex is thin and smooth, but the large volume of subcortical white matter suggests extensive association connections between cortical areas. The allocortical areas are small in volume (Reep et al., 2007), with a tiny piriform cortex and small hippocampal formation.Table 1 below summarises the correlation of cortical areas identified by Sarko and Reep with those in the current mapping. Note that some cortical regions extend across more than one lobe. Table 1 below summarises the correlation of cortical areas identified by Sarko and Reep with those in the current mapping. Note that some cortical regions extend across more than one lobe.
Cluster cortex is named for the presence of Rindenkerne (abbreviated Rk, translated from German as “cortical nuclei”), which are clumps of microneurons in the deepest cortical layer (Johnson et al., 1994). In the specimen depicted, these clumps are most obvious in CL1 and CL2 (see Plates 3 to 5), which are probably the parts of the primary somatosensory cortex concerned with processing trigeminal nerve inputs from the perioral bristles (Sarko and Reep, 2007; Reep et al., 1989; 2001; Reyes et al., 2015).
Lissencephaly in sirenians appears to be associated with limited expansion of the upper layers of the cerebral cortex (layers 2 to 4) in these mammals (Charvet et al., 2016), in contrast to the brains of primates and carnivores where gyrencencephaly (folding of the cerebral cortex) and upper cortical layer expansion have proceeded in concert.
The Manatee Somatosensory System
The mammalian somatosensory system for touch, vibration, joint position, pain and temperature consists of a series of connected neuronal groups in the spinal cord, brainstem, thalamus and cerebral cortex. Somatosensation from the body is transmitted through dorsal column and spinothalamic pathways in the spinal cord. Dorsal column pathways for sensation from the body and limbs include tracts in the spinal cord which terminate in the gracile (Gr) and cuneate (Cu) nuclei of the medulla, which in turn project to the ventral posterior lateral thalamic nucleus (VPL), which in turn projects to the body and limb region of primary somatosensory cortex (S1). Touch, pain and temperature from the head is carried by the trigeminal nerve (5n) to the trigeminal sensory nuclei of the brainstem (Pr5, Sp5O, Sp5I, Sp5C), which project to the ventral posterior medial thalamic nucleus (VPM), which in turn projects to the face region of S1.
Somatosensory nuclei (Gr, Cu, Pr5, Sp5O, Sp5I, Sp5C, VPL, VPM) are all very large in the manatee, consistent with the behavioural importance of somatosensation from both the head and body in manatees (Sarko et al., 2007). The trigeminal somatosensation from the head is mainly associated with the peri-oral bristles and the sensory demands of oripulation (see below). Somatosensation from body and limbs is probably associated with the processing of hydrodynamic information (i.e. vibration of body hairs by water flow) to control swimming (Gaspard et al., 2017).
Functional Neuroanatomy of Oripulation
Florida manatees have modified vibrissae (peri-oral bristles) that can be moved to manipulate vegetation for ingestion (oripulation) and to explore the environment (Marshall et al., 2007). The facial motor nucleus (7DM, 7VM, 7DI, 7VI, 7DL, 7L) controls facial musculature and is situated in the rostral brainstem. Based on facial motor nucleus musculotopy seen in rodents and primates, it is likely that the vibrissal musculature is controlled by motorneurons in the lateral subnucleus and this is consistent with the enlargement of that subnucleus in the manatee (please see Plate 14).
Acknowledgements
I would like to thank Elizabeth Lockett of the National Museum of Health and Medicine in Silver Springs, Maryland, USA for the opportunity to study the marine mammal brains in the NMHM collection and for use of the Epson 11000 scanner.
References
Bullock TH, Domning DP, Best RC (1980) Evoked brain potentials demonstrate hearing in a manatee (Trichechus inunguis). J Mammal 61, 130-133.
Charvet CJ, Reep RL, Finlay BL (2016) Evolution of cytoarchitectural landscapes in the mammalian isocortex: sirenians (Trichechus manatus) in comparison to other mammals. J Comp Neurol 524, 772-782.
Gaspard JC III, Bauer GB, Mann DA, Boerner K, Denum L, Frances C, Reep RL (2017) Detection of hydrodynamic stimuli by the postcranial body of Florida manatees (Trichechus manatus latirostris). J Comp Physiol A 203, 111-120.
Johnson JI, Kirsch JAW, Reep RL, Switzer RC III (1994) Phylogeny through brain traits: more characters for the analysis of mammalian evolution. Brain Behav Evol 43, 319-347.
Marshall CD, Vaughn SD, Sarko DK, Reep RL (2007) Topographical organization of the facial motor nucleus in Florida manatees (Trichechus manatus latirostris). Brain Behav Evol 70, 164-173.
Pirlot P, Kamiya T (1985) Qualitative and quantitative brain morphology in the sirenian Dugong dugong Erxl. Z zool Syst Evolut. -forsch 23, 147-155.
Rainey WE, Lowenstein JM, Sarich VM, Magor DM (1984) Sirenian molecular systematics – including the extinct Stellar’s sea cow (Hydrodamalis gigas). Naturwissench 71, 586-588.
Reep RL, O’Shea TJ (1990) Regional brain morphometry and lissencephaly in the Sirenia. Brain Behav Evol35, 185-194.
Reep RL, Johnson JI, Switzer RC III, Welker WI (1989) Manatee cerebral cortex: cytoarchitecture of the frontal region in Trichechus manatus latirostris. Brain Behav Evol 34, 365-386.
Reep RL, Stoll ML, Marshall CD, Homer BL, Samuelson DA (2001) Microanatomy of facial vibrissae in the Florida manatee: the basis for specialized sensory function and oripulation. Brain Behav Evol 58, 1-14.
Reep RL, Finlay BL, Darlington RB (2007) The limbic system in mammalian brain evolution. Brain Behav Evol70, 57-70.
Reyes LD, Stimpson CD, Gupta K, Raghanti MA, Hof PR, Reep RL, Sherwood CC (2015) Neuron types in the presumptive somatosensory cortex of the Florida manatee (Trichechus manatus latirostris). Brain Behav Evol86, 210-231.
Sarko DK, Reep RL (2007) Somatosensory areas of manatee cerebral cortex: histochemical characterization and functional implications. Brain Behav Evol 69, 20-36.
Sarko DK, Johnson JI, Switzer RC III, Welker WI, Reep RL (2007) Somatosensory nuclei of the manatee brainstem and thalamus. Anat Rec 290, 1138-1165.
Seiffert ER (2007) A new estimate of afrotherian phylogeny based on simultaneous analysis of genomic, morphological and fossil evidence. BMC Evol Biol 7, 224.
Vianna JA, Bond RK, Caballero S, Giraldo JP, Lima RP, Clark A, Marmontel M, Morales-Vela B, de Souza MJ, Parr L, Rodríguez-Lopez MA, Mignucci-Giannoni AA, Powell JA, Santos FR (2006) Phylogeography, phylogeny and hybridization in trichechid sirenians: implications for manatee conservation. Mol Ecol 15, 433-447.
Nissel-stained Sections through the brain of the Florida Manatee.
Below is a grid of the frontal Nissl-stained sections through the brain from Plate 1 to Plate 18. Please see next section for larger image.
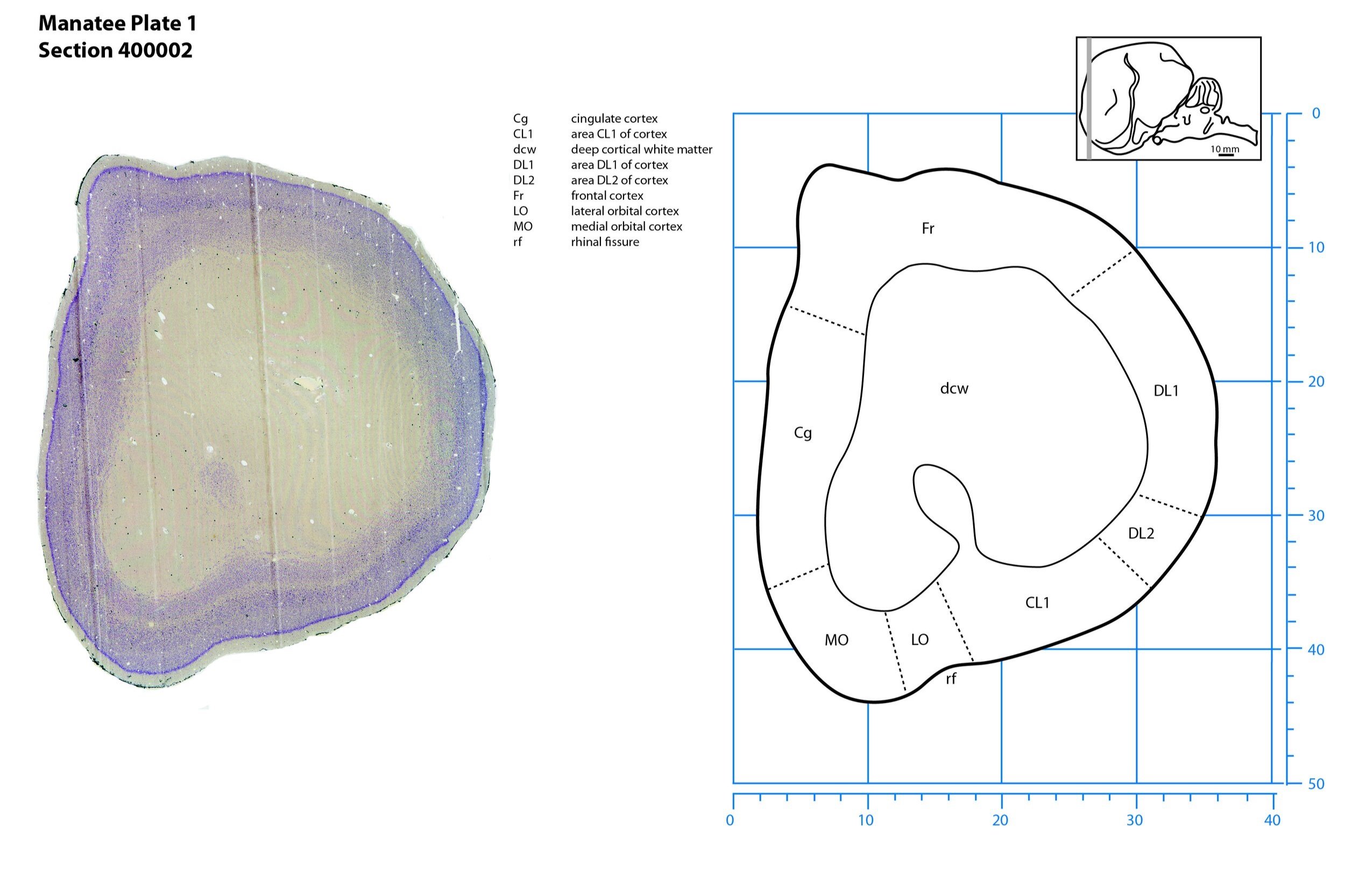
Plate 1 Frontal Nissl-stained section through the brain of the Florida manatee (section 400002 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the rostral pole of the telencephalon. The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
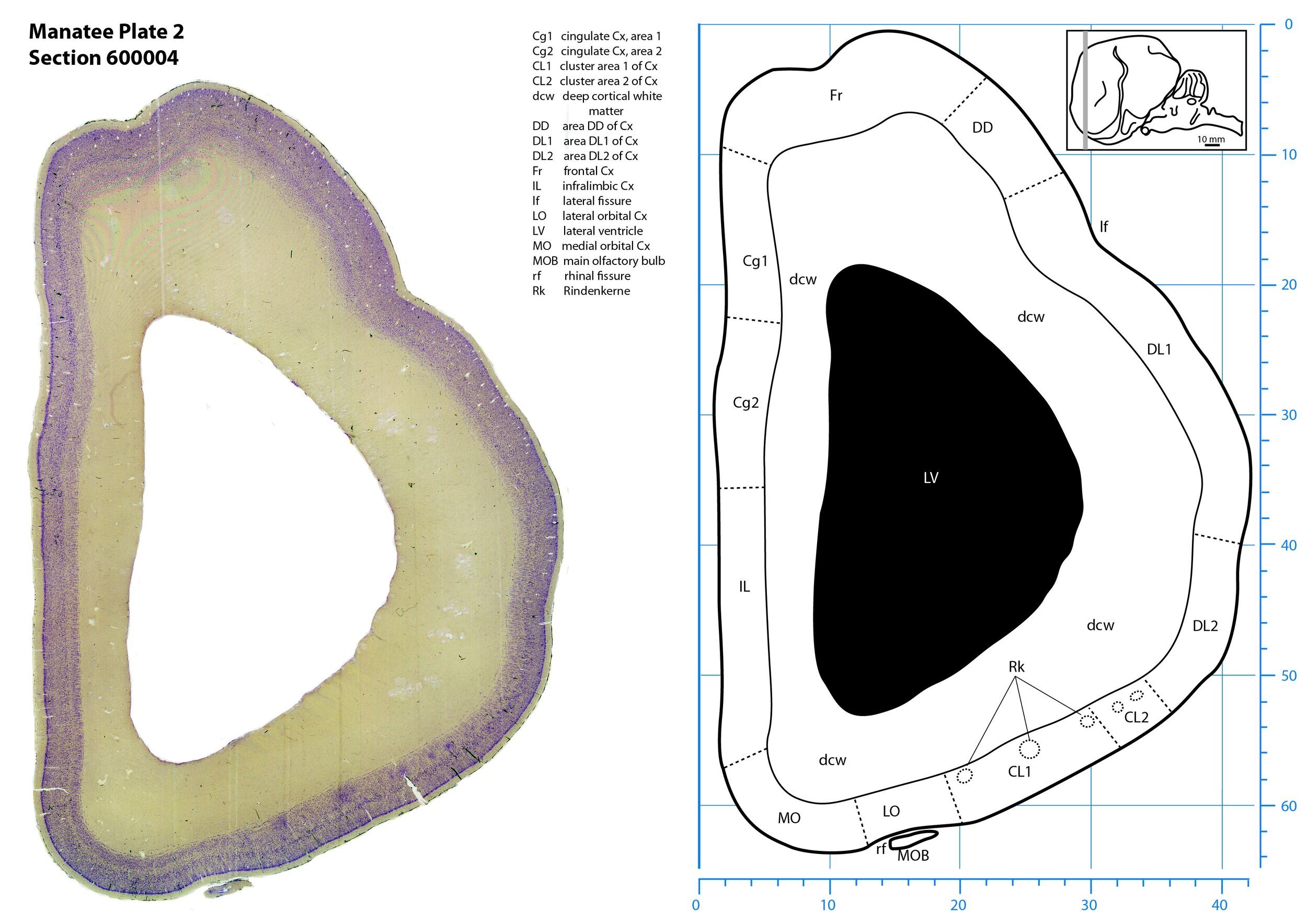
Plate 2 Frontal Nissl-stained section through the brain of the Florida manatee (section 600004 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the rostral main olfactory bulb (MOB). The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
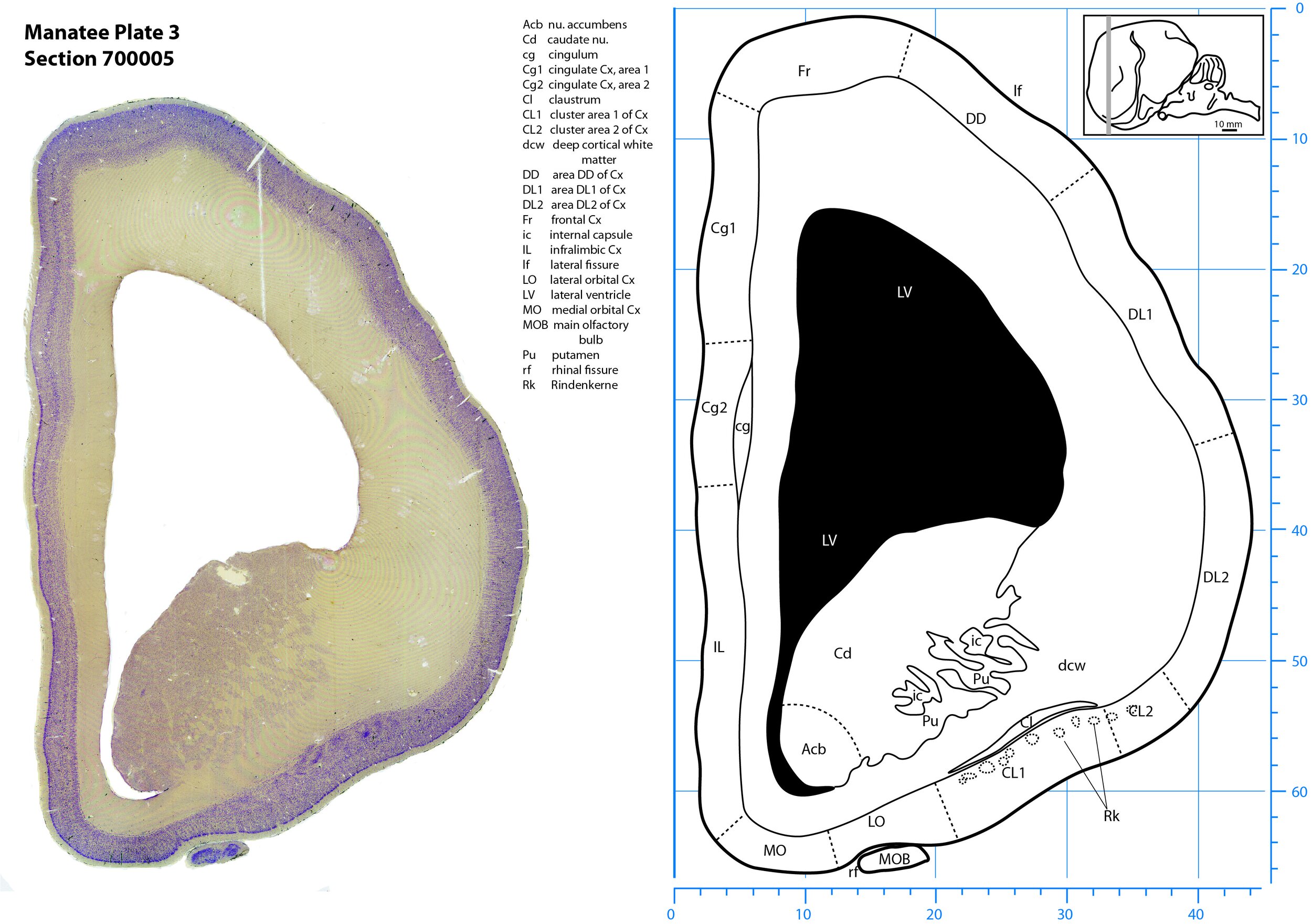
Plate 3 Frontal Nissl-stained section through the brain of the Florida manatee (section 700005 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the head of the caudate nucleus (Cd). The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
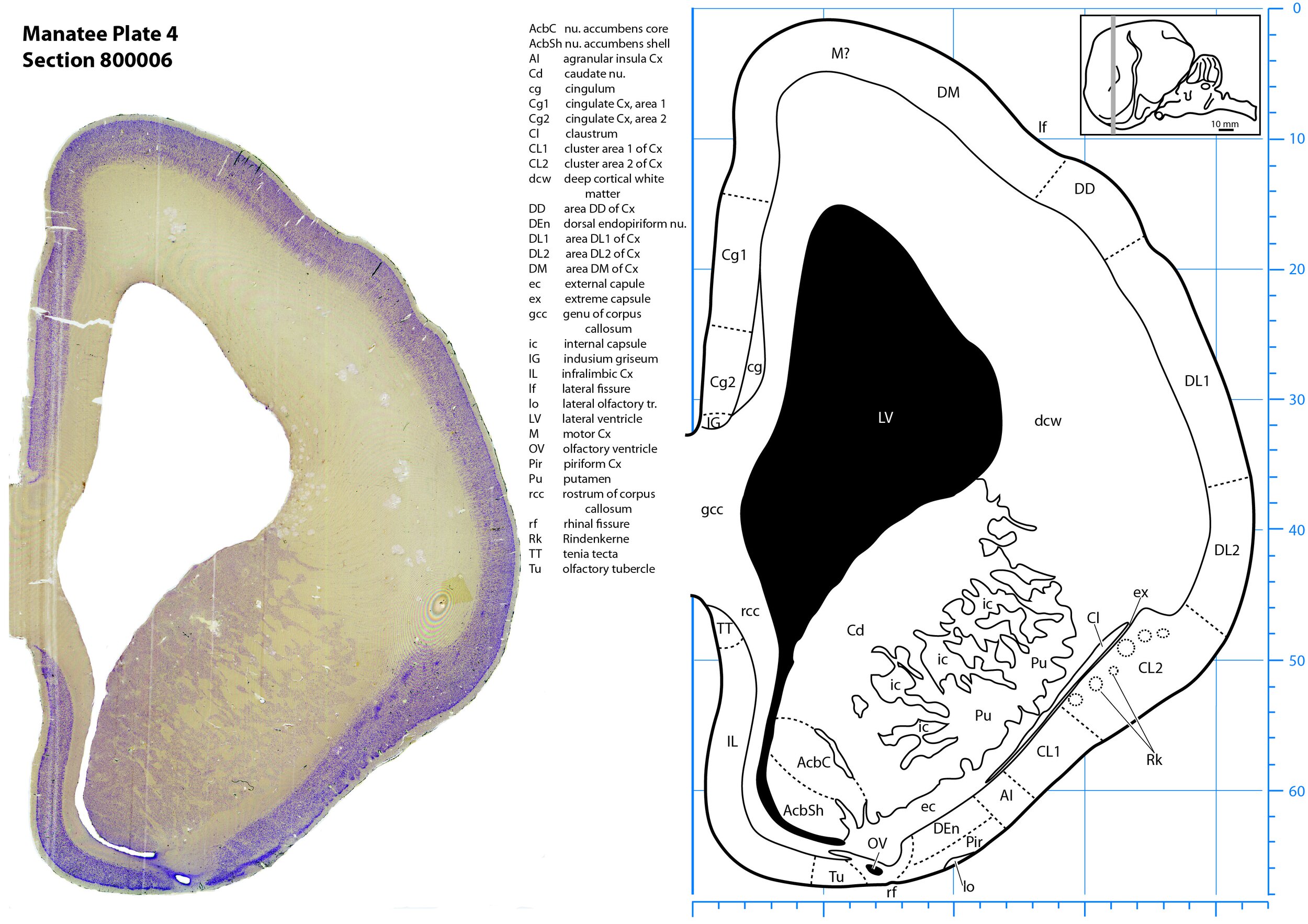
Plate 4 Frontal Nissl-stained section through the brain of the Florida manatee (section 800006 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the genu of the corpus callosum (gcc). The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
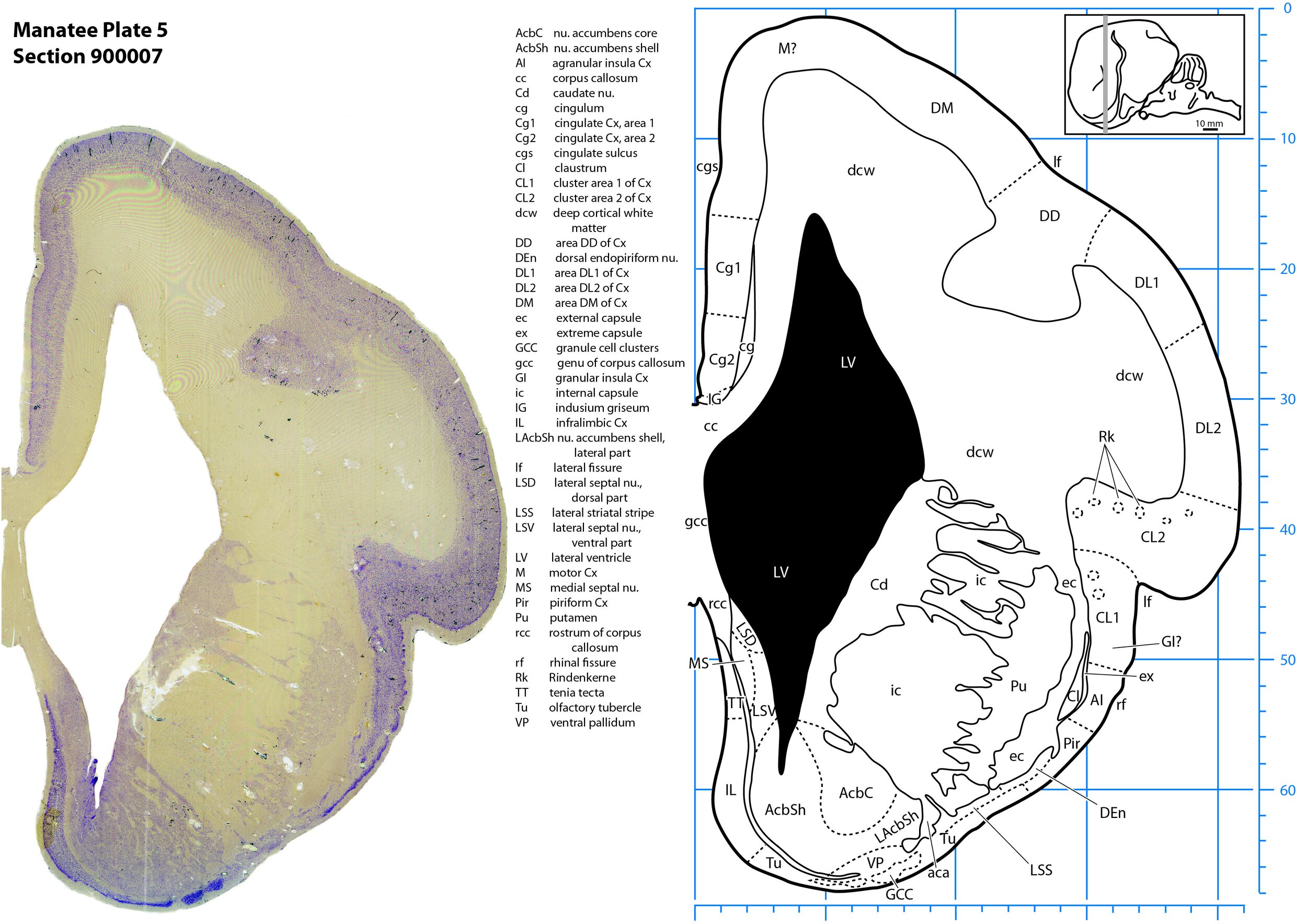
Plate 5 Frontal Nissl-stained section through the brain of the Florida manatee (section 900007 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the septal nuclei (MS, LSD, LSV). The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
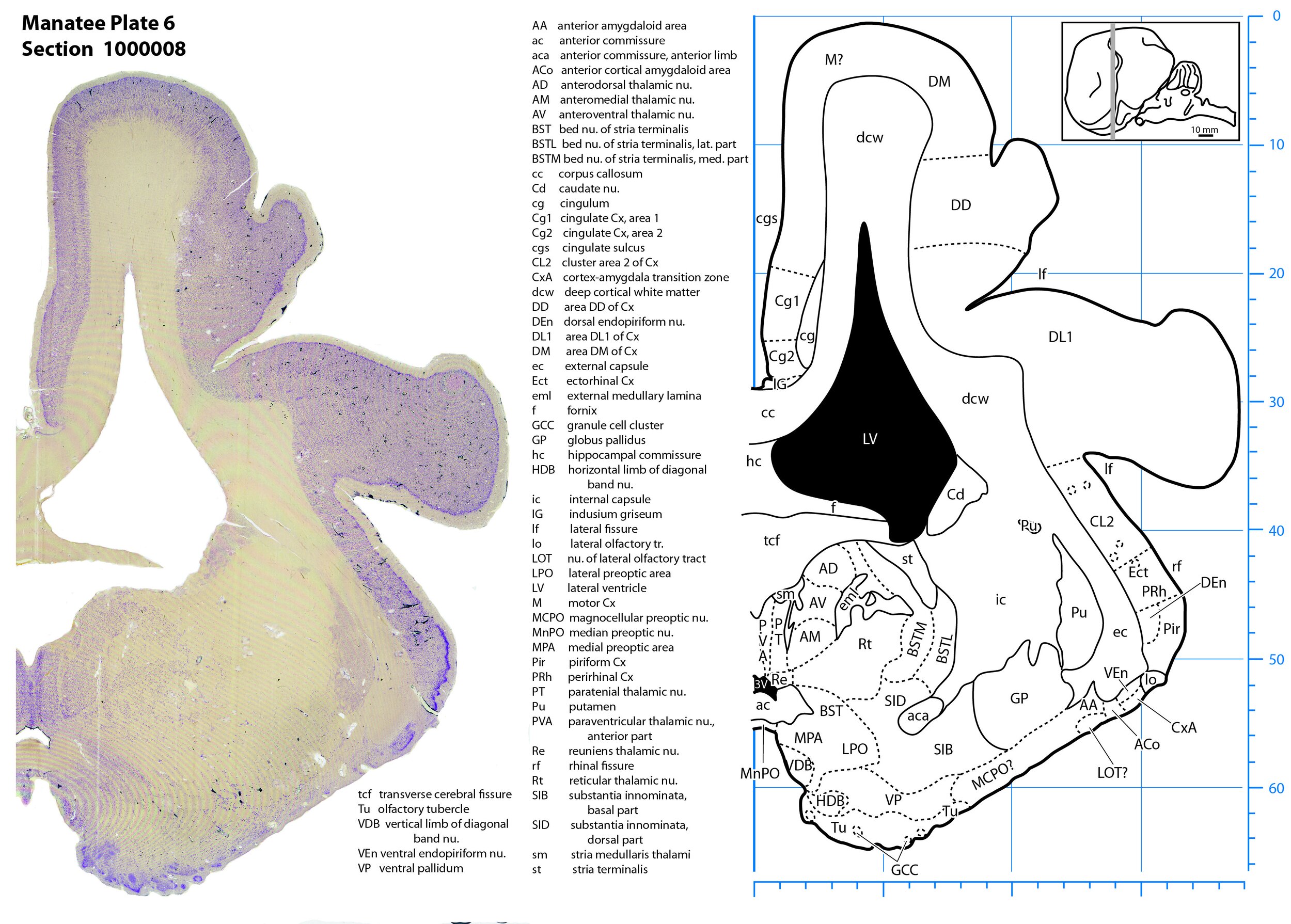
Plate 6 Frontal Nissl-stained section through the brain of the Florida manatee (section 1000008 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the anterior thalamus (Ad, AV, AM). The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
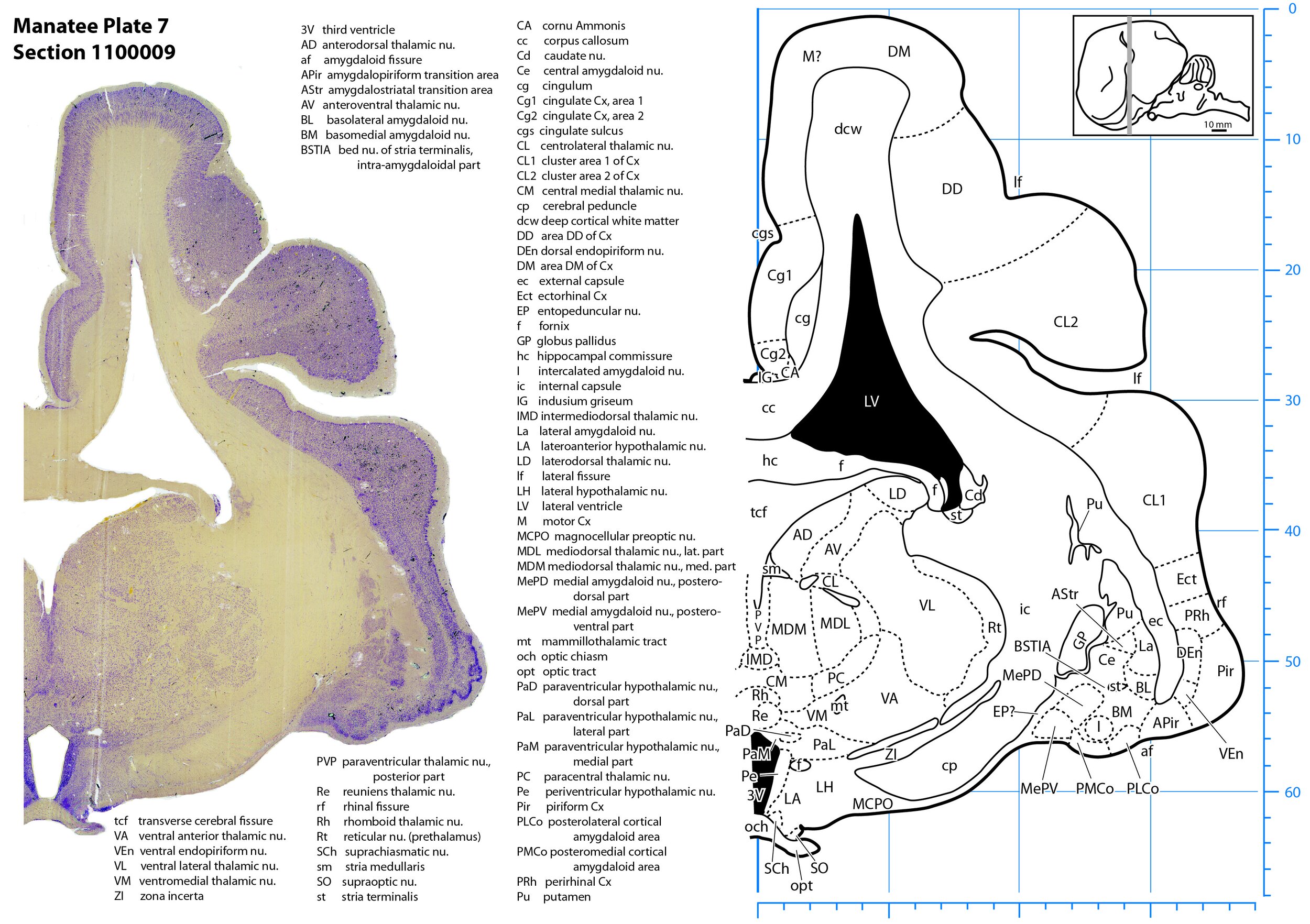
Plate 7 Frontal Nissl-stained section through the brain of the Florida manatee (section 1100009 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the optic chiasm. The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
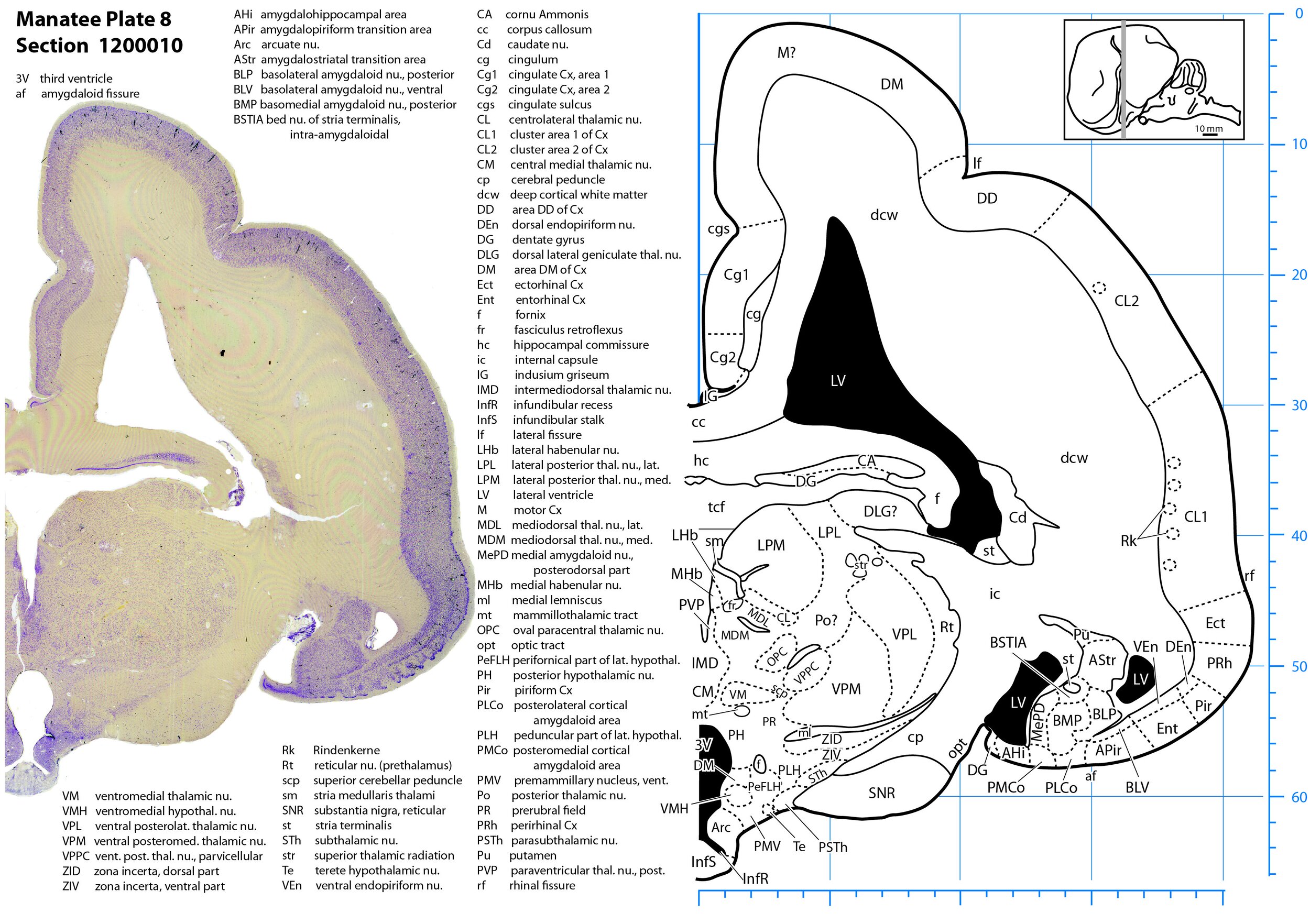
Plate 8 Frontal Nissl-stained section through the brain of the Florida manatee (section 1200010 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the pituitary stalk. The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
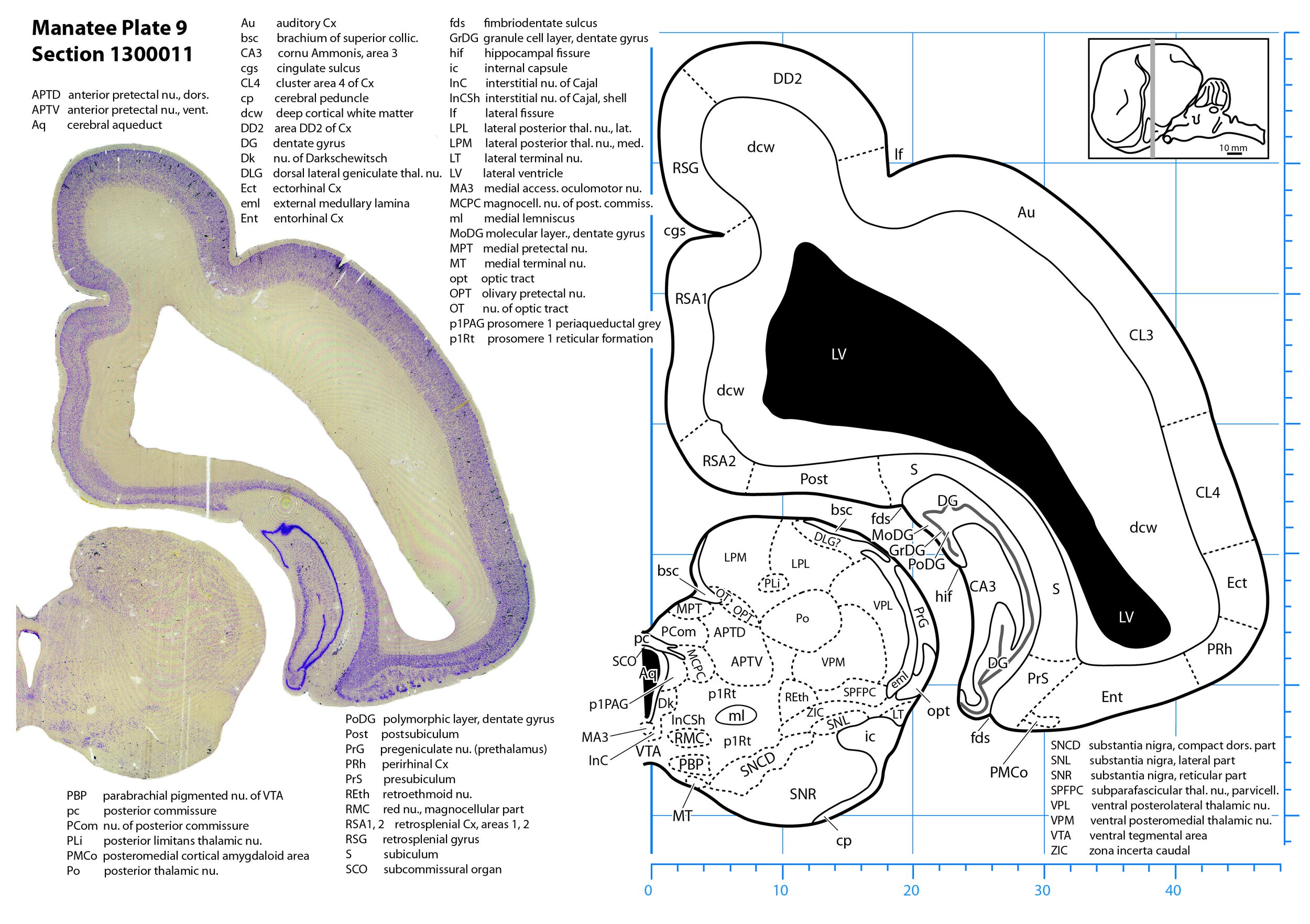
Plate 9 Frontal Nissl-stained section through the brain of the Florida manatee (section 1300011 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the posterior thalamus. The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
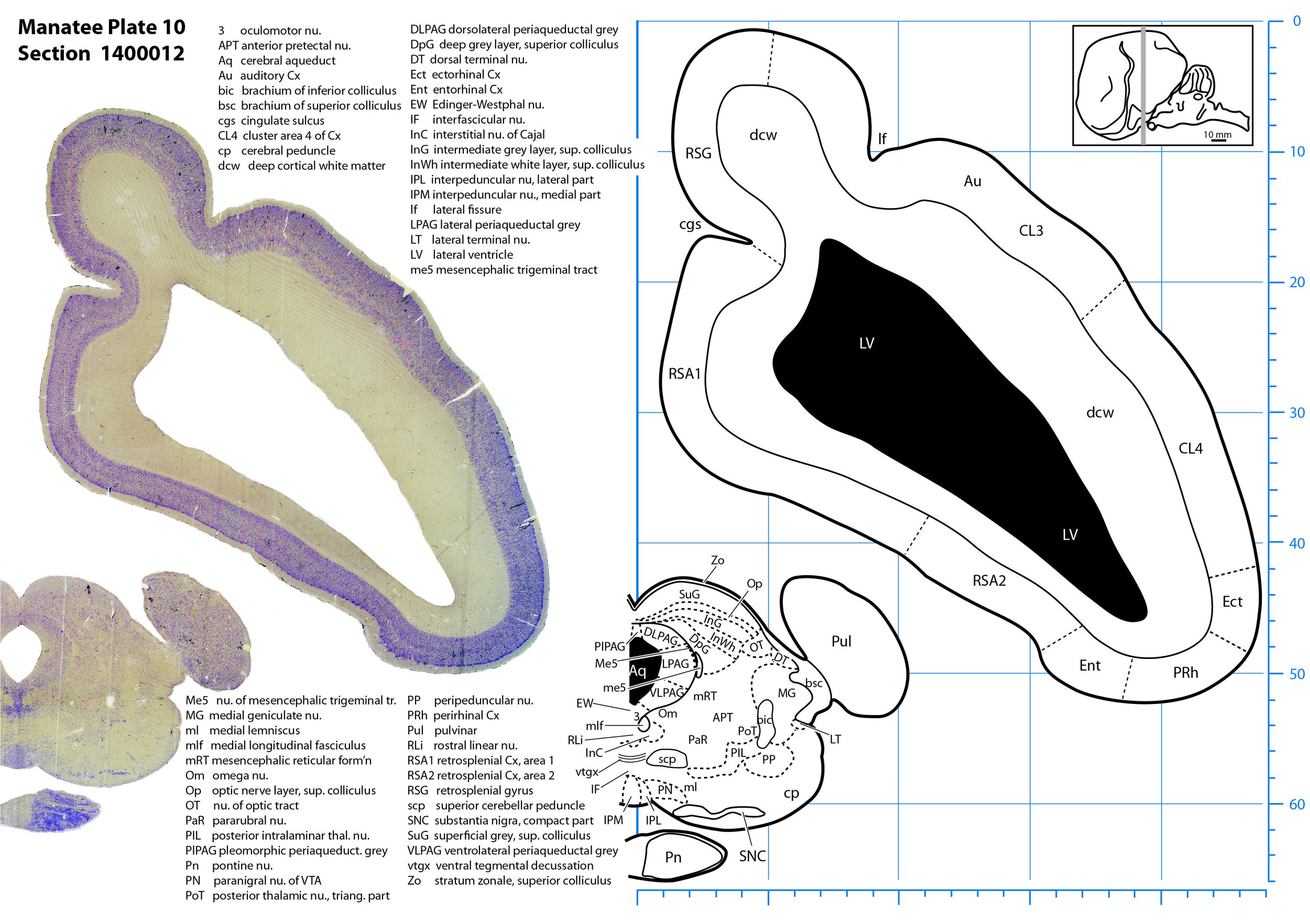
Plate 10 Frontal Nissl-stained section through the brain of the Florida manatee (section 1400012 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the superior colliculus. The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
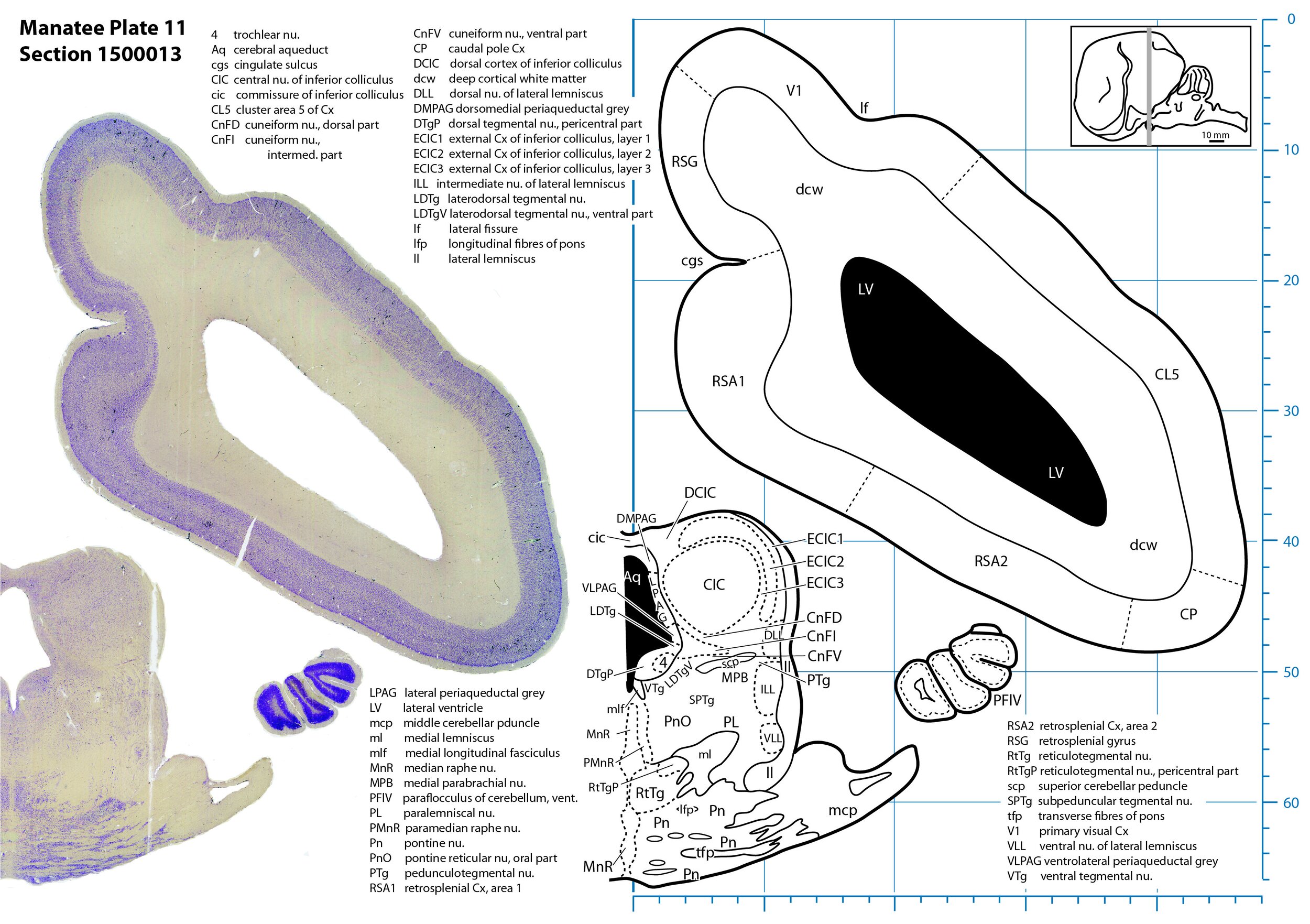
Plate 11 Frontal Nissl-stained section through the brain of the Florida manatee (section 1500013 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the inferior colliculus. The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
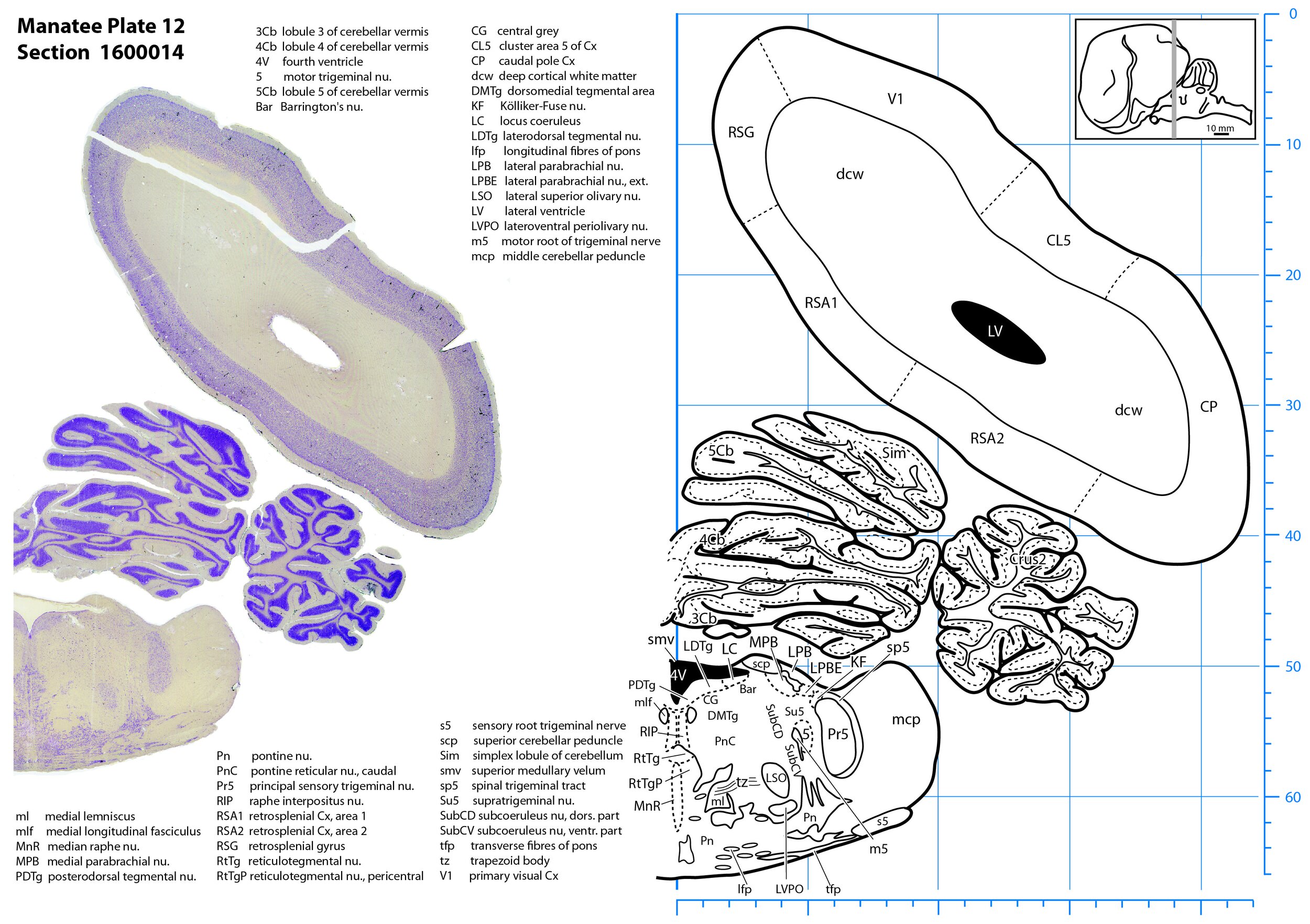
Plate 12 Frontal Nissl-stained section through the brain of the Florida manatee (section 1600014 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the pontine nuclei. The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
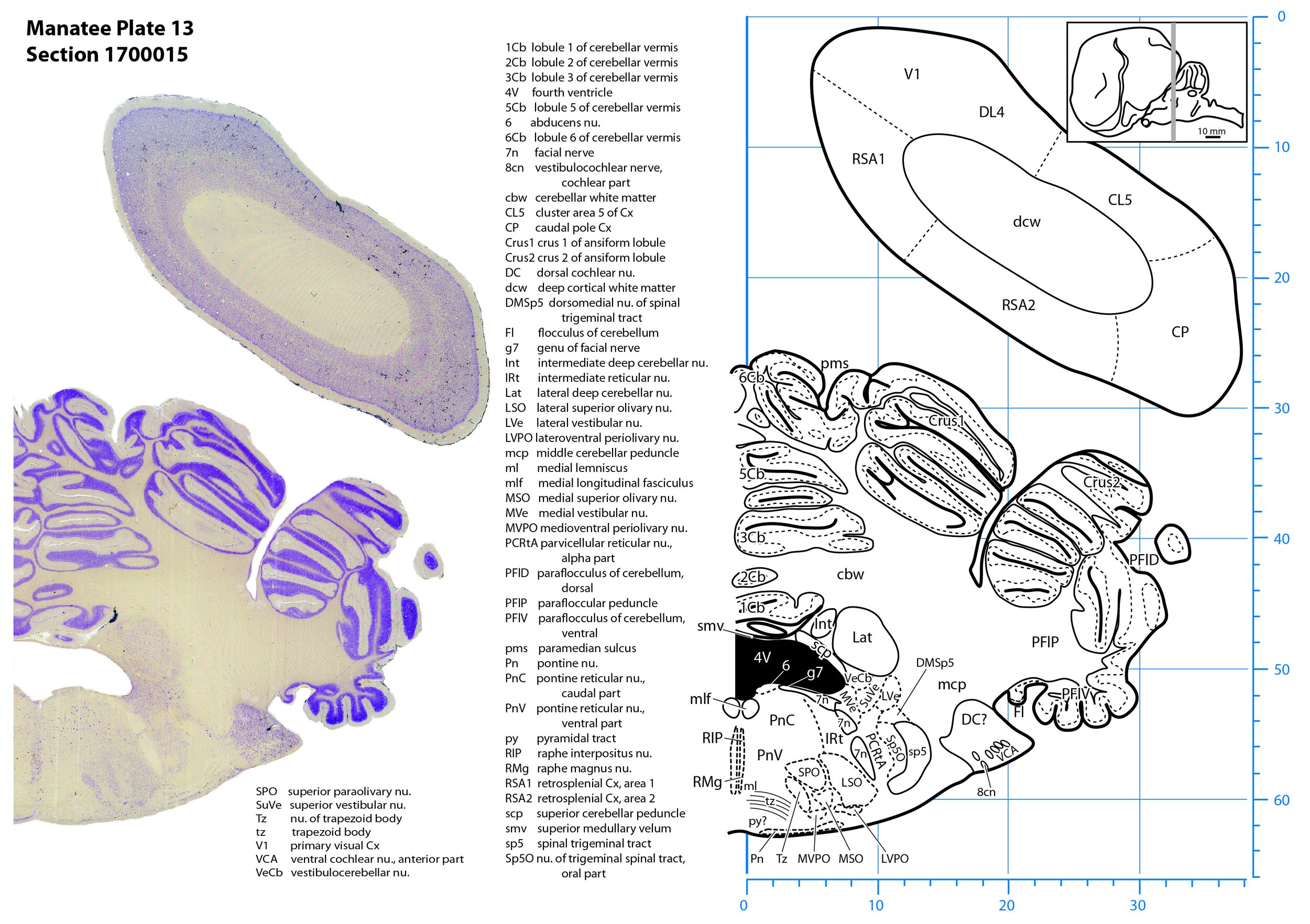
Plate 13 Frontal Nissl-stained section through the brain of the Florida manatee (section 1700015 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the superior olivary nuclear complex. The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
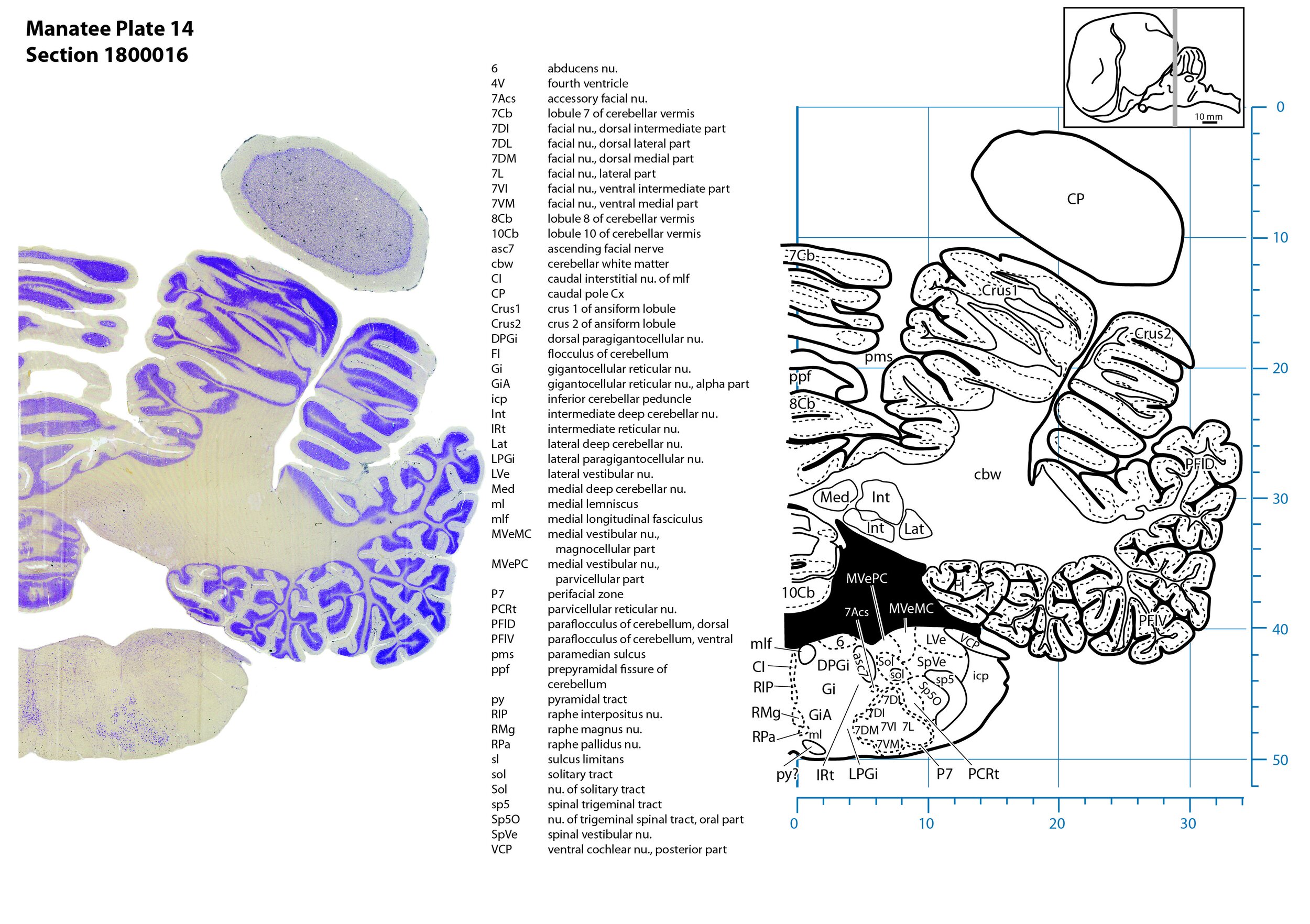
Plate 14 Frontal Nissl-stained section through the brain of the Florida manatee (section 1800016 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the facial motor nucleus. The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
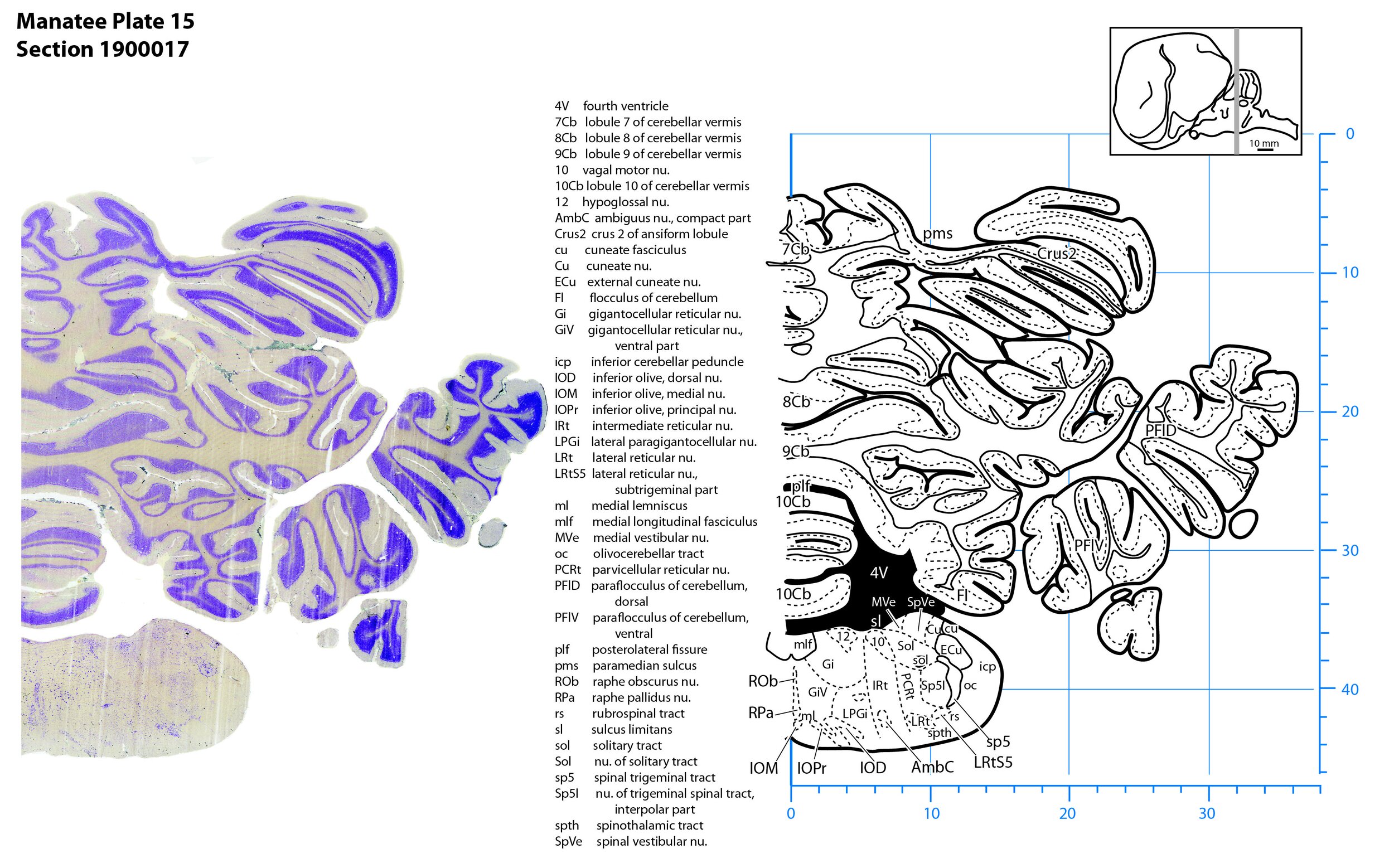
Plate 15 Frontal Nissl-stained section through the brain of the Florida manatee (section 1900017 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the rostral inferior olivary nuclear complex. The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
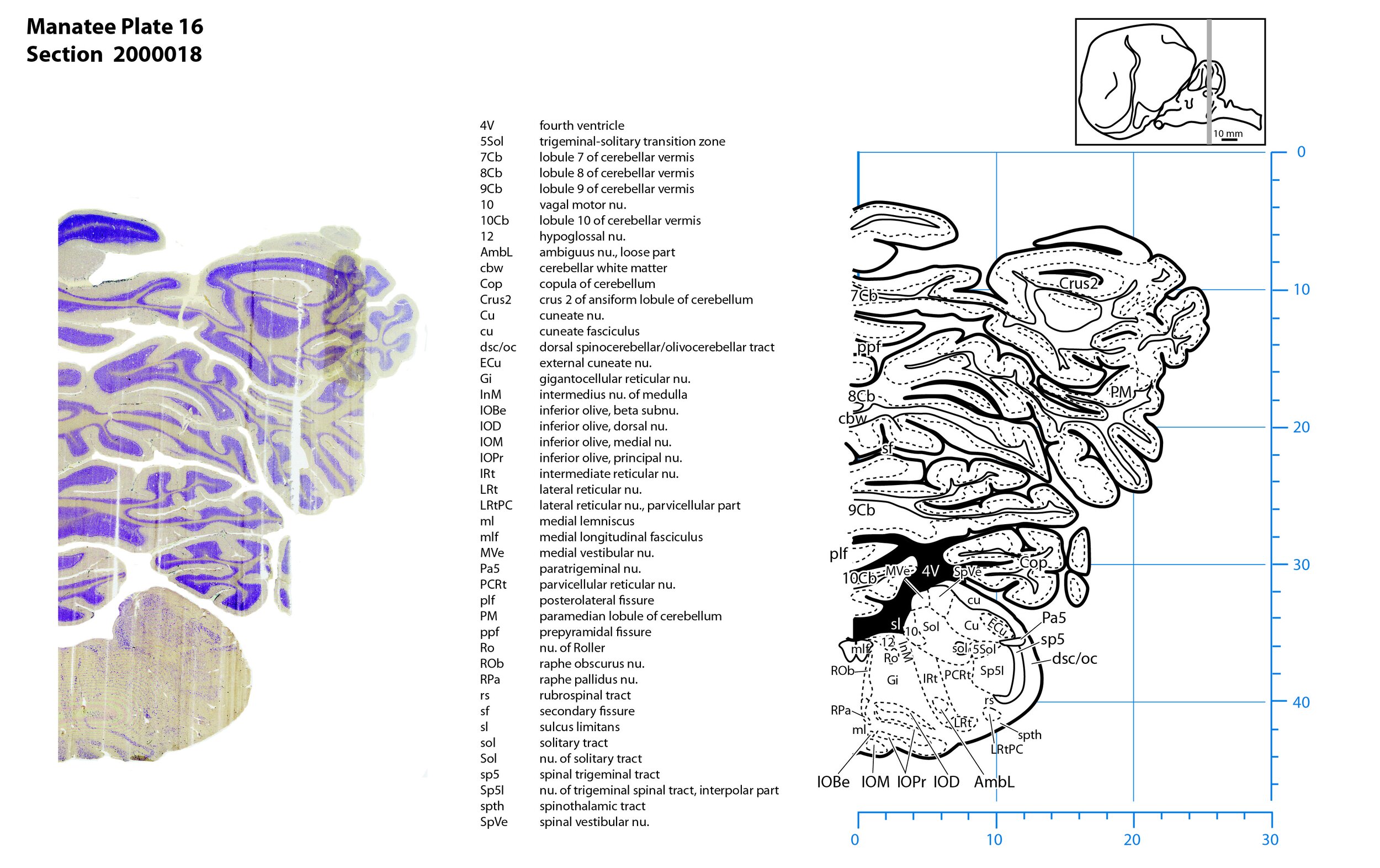
Plate 16 Frontal Nissl-stained section through the brain of the Florida manatee (section 2000018 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the middle inferior olivary nuclear complex. The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
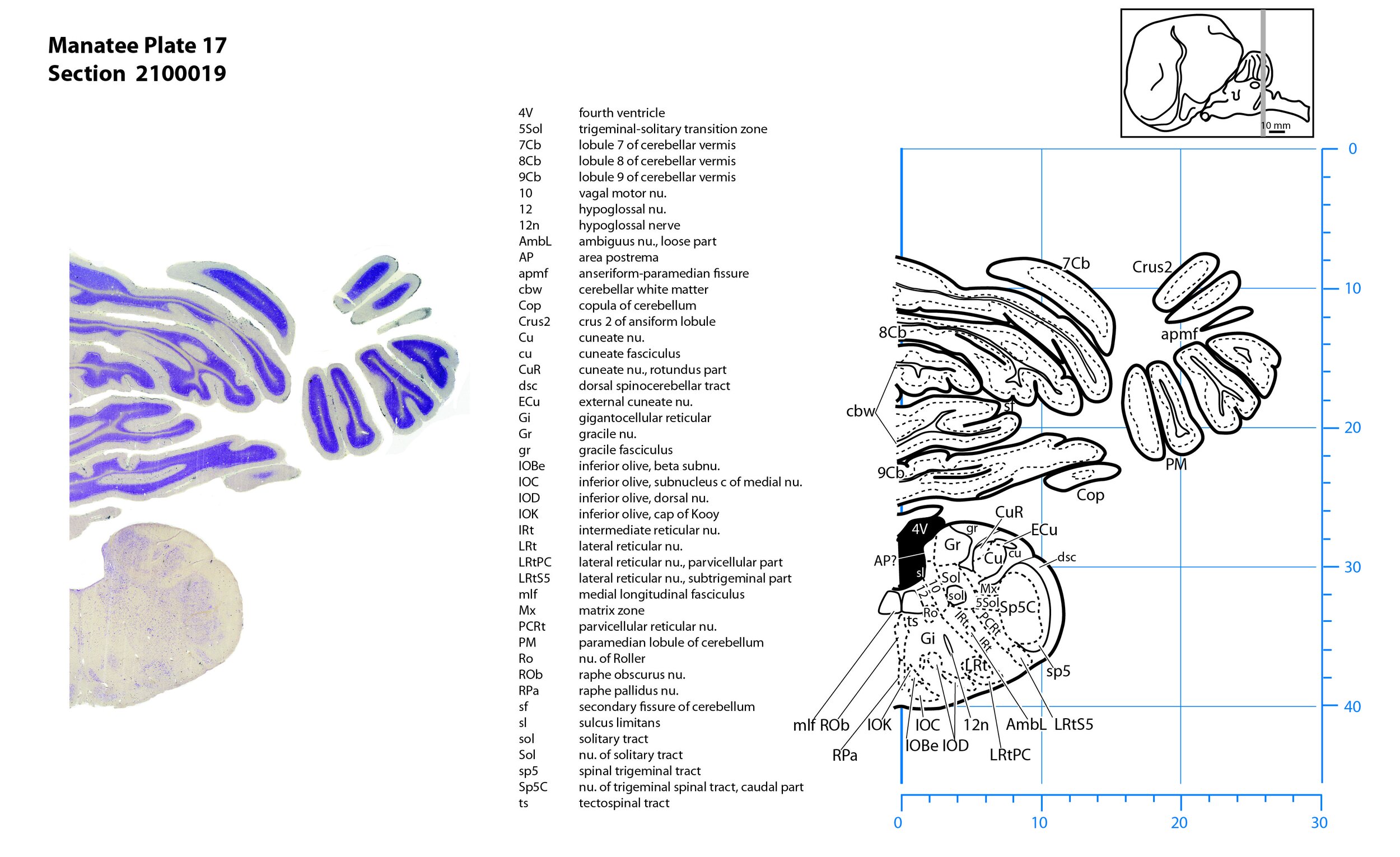
Plate 17 Frontal Nissl-stained section through the brain of the Florida manatee (section 2100019 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the caudal inferior olivary nuclear complex. The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.
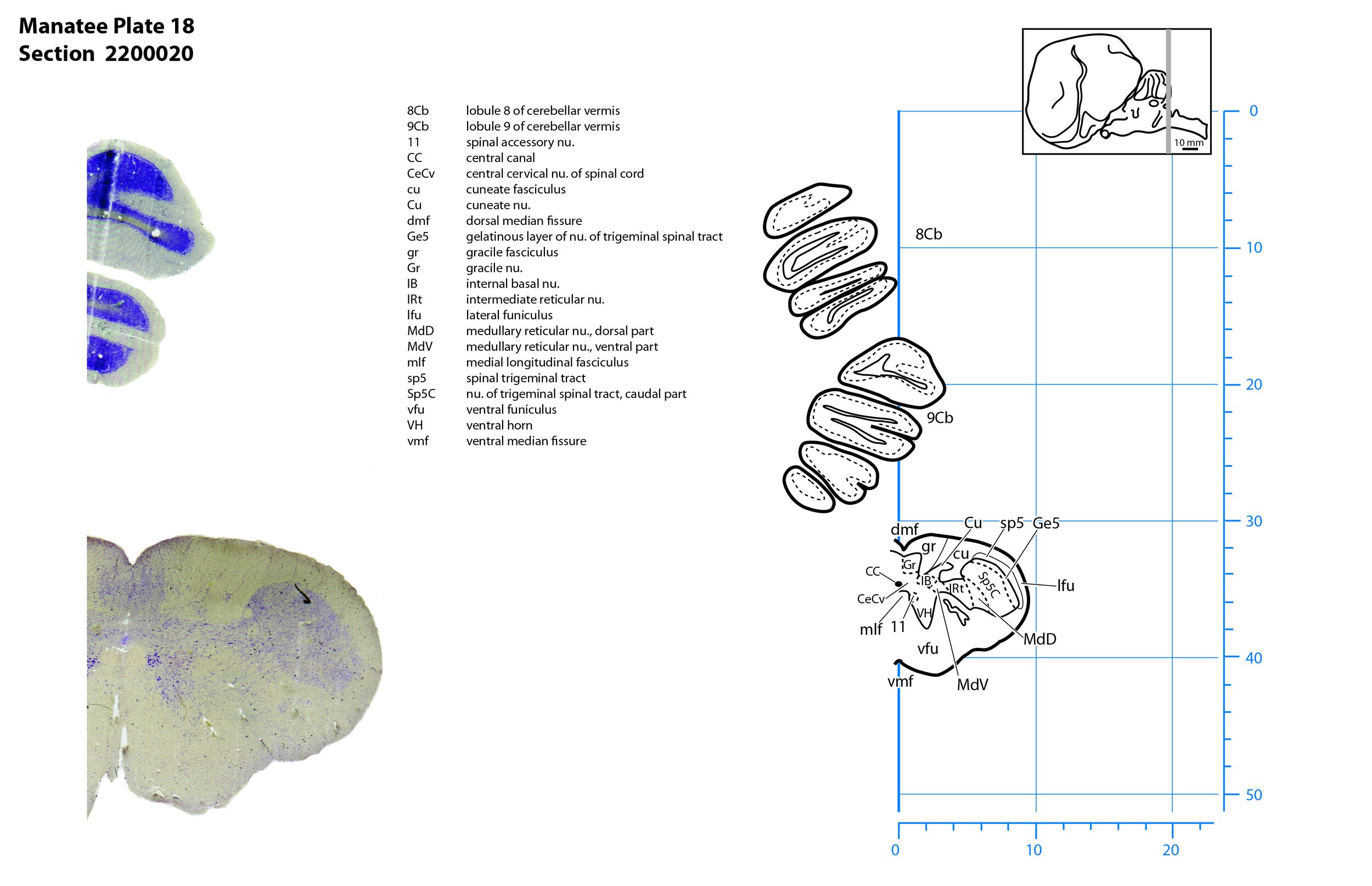
Plate 18 Frontal Nissl-stained section through the brain of the Florida manatee (section 2200020 of Welker Trichechus manatus, 84-49, held at NMHM, Maryland USA) at the level of the spinomedullary junction. The finder diagram in the top right-hand corner shows the position of the section (grey line) in the rostrocaudal sequence through the brain. Scale is in mm.

